Liver fibrosis is a major characteristic of most chronic liver diseases which leads to accumulation of extracellular matrix (ECM) proteins. Hedgehog (Hh) pathway activated by Gli genes participated in the pathogenesis of liver fibrosis. However, the regulatory role of miR-125b in liver fibrosis via targeting Gli genes remains unknown.
Materials and methodsRT-qPCR and western blot were employed to the expression levels of mRNA and protein, respectively. The fibrosis level of liver tissue was determined by Masson's trichrome staining. The interaction between miR-125b and Gli3 was tested by luciferase reporter assay. In addition, LX2 cells were activated and CCl4-induced rat model was used in this study.
ResultsmiR-125b was significantly declined in serum samples of the clinical liver fibrosis patient, activated LX2 cells and the liver tissues of the CCl4-induced rat model. Furthermore, in cellular level, the alpha-smooth muscle actin (α-SMA) and Albumin expressions were ascending and descending in LX2 cells, respectively, with the decline of miR-125b. However, when transfecting with miR-125b mimic, the expressions of α-SMA and Albumin was reversed and Gli3 expression was notably repressed in LX2 cells. The target interaction between miR-125b and Gli3 was determined by dual-luciferase assays. It was further discovered that the changes of α-SMA, Albumin, and Gli3 were similar to the expression trend in LX2 cells with miR-125b mimic transfection.
ConclusionThese results suggested that miR-125b might be protective against liver fibrosis via regulating Gli3 and it might be a promising target in the development of novel therapies to treat pathological fibrotic disorders.
Many chronic liver diseases, such as hepatitis B (HBV) or C (HCV) infection, excess alcohol consumption, non-alcoholic steatohepatitis (NASH), metabolic syndrome, and autoimmune hepatitis, can lead to hepatic fibrosis, which is characterized by the excessive accumulation of extracellular matrix (ECM) and disturbance of the lobular structure which eventually result in liver cirrhosis, hepatic failure and primary liver cancer [1]. Various cell types are considered sources of ECM in liver fibrosis [2,3]; however, among these, hepatic stellate cells (HSCs) are the major ECM-producing cells, which are quiescent in healthy livers and are stimulated by profibrotic cytokines during the process of liver fibrogenesis [4]. Despite many researchers have made impressive progress in our understanding of the mechanisms that underlie the pathogenesis of liver fibrosis in the past two decades, treatments available for this disease are still limited [5,6]. Currently, the limited available curative treatment options mainly contain antiviral therapy for chronic HBV and HCV, weight loss and exercise for NASH or liver transplantation for hepatonecrosis [7–9]. Moreover, a lot of patients with liver fibrosis are diagnosed at intermediate or advanced disease stages and do not respond to the above treatments, thereby the satisfactory curative approaches are often not feasible [10]. Based on the potential mechanism of HSC activation in the progression of liver fibrosis, understanding the mechanism of HSCs activation might be an effective anti-fibrotic therapy in the future.
microRNAs (miRNAs), single-stranded, endogenous, small non-coding RNA molecules of 20–22 nucleotides in length, act as negative regulators of gene expression by inhibiting protein translation or inducing mRNA degradation [11,12]. Growing evidence has demonstrated that mammalian miRNAs are involved in various biological processes, such as differentiation, proliferation, immune responses, oxidative stress resistance, and carcinogenesis [13]. Moreover, it was well known that the abnormal expression of miRNAs has been found to be closely associated with human liver diseases, such as liver cancer, autoimmune liver disease and viral hepatitis [14].
Additionally, most studies have been conducted to investigate that miRNAs can regulate the activation of HSCs in liver fibrosis through targeting profibrotic gene expression [15,16]. For example, miR-378 limited activation of HSCs and liver fibrosis by suppressing GLI family zinc finger 3 (Gli3) expression [17]; miR-9 hindered hepatic fibrosis by reducing the activation and proliferation of HSCs by directly targeting multi-drug resistance-associated protein 1 (MRP1/ABCC1) [18]; miR-214 promotes hepatic stellate cell activation and liver fibrosis by suppressing Sufu expression [19]. miR-30a inhibited the epithelial-mesenchymal transition (EMT) process, at least in part, via reduction of Snai1, leading to the suppression of HSC activation in liver fibrosis [20]. Recently, miR-125b has been reported to be implicated in a wide variety of physiological processes, including cell differentiation, immune response, pain signaling, cell metabolism [21,22]. Furthermore, the differential expression of miR-125b has also been discovered in hepatocellular carcinoma (HCC), HBV-related liver diseases, etc. [23,24]. Nevertheless, the roles of miR-125b in liver fibrosis are still unclear.
Hedgehog (Hh) signaling, a critically important signaling pathway in hepatic fibrogenesis, contributes to the process of HSC activation and their ability to synthesize ECM components [24]. Thus, it indicated that the suppression of Hh signaling could impede the formation of liver fibrosis. Accumulating evidence has demonstrated that Gli3 can function as a Hh signaling-independent transcriptional activator [25,26]. Moreover, the bioinformatic analysis also showed that there might be a potential target interaction between miR-125b and Gli3. Therefore, in this study, we aimed to investigate the expressions of miR-125b in clinical, animal and cellular samples, verify the interaction between miR-125b and Gli3, and further explore the role of miR-125b in the process of liver fibrosis.
2Materials and methods2.1Patients and sample collectionA total of 10 cases of liver fibrosis that were diagnosed between August 2015 and January 2017 at The First People's Hospital of Kunming City and 10 cases of healthy persons were enrolled. The inclusion criteria of patients with liver fibrosis were as follows: no preoperative chemotherapy, no chronic basic disease and no long history of medication, and the cause of liver fibrosis was alcohol liver injury. Meanwhile, normal controls were not suffering from any other diseases. Fasting venous blood samples were harvested by trained laboratory technicians. Generally, five milliliters of peripheral blood were kept at room temperature for 1h and then cellular components were removed by two consecutive centrifugation steps (3000rpm for 10min at 4°C and 4000rpm for 3min at 4°C, respectively). Finally, serum in the upper layer was aspirated, aliquoted and stored at −80°C until testing.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of The First People's Hospital of Kunming City and with the 1964 Helsinki Declaration. Moreover, written informed consent was obtained from all participants or their families.
2.2Cell cultureHuman normal hepatocyte, including AML12 cell and LO2 cell, and hepatic stellate cell LX2 purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), were cultured at 37°C in a humidified atmosphere containing 5% CO2 with Dulbecco's modified Eagle's medium (DMEM; Gibco, USA) containing 2mmol/L l-glutamine, 100U/mL penicillin, 100mg/mL streptomycin, and 10% fetal bovine serum (FBS; Gibco, USA). It took about 2 days for the cells to grow to 80% confluence before the culture media were subcultured and replaced.
THP-1 cells purchased from American Type Culture Collection (ATCC) were cultivated in RPMI-1640 medium (Gibco, USA) with the additions of 10% FBS, and antibiotics (100IU/mL penicillin and 100mg/mL streptomycin) and kept at 37°C under 5% CO2. THP-1 cells were differentiated into macrophages over 48h in RPMI 1640 medium containing 5–25ng/mL PMA, according to a previously published method [27] Then, LX2 cell lines with the characteristics of an activated hepatic stellate cell (HSC) phenotype were pre-cocultured with the treated THP-1 cells and challenged with LPS (1μg/mL) as described by Vincenzo [28]. After 0h, 6h, 12h, 24h and 48h, the cells were collected for subsequent analysis.
293T cells obtained from ATCC were grown in RPMI-1640 supplemented with 10% FBS, l-glutamine, 1% Penicillin/Streptomycin and maintained in the humidified incubator at 37°C with 5% CO2 for the dual luciferase assay.
2.3RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)Total RNA, containing miRNA, was extracted with TRIzol reagent (Invitrogen, USA) according to the manufacturer's protocol. For mature miR-125b expression analysis, 1mg of total RNA was converted to complementary DNA (cDNA) using the PrimeScript RT Master Mix Kit (Takara, Japan) with a miR-125b-specific primer. Subsequently, PCR reaction was performed using the SYBR Green PCR Kit (Takara, Japan) according to the manufacturer's instructions on an ABI 7500 thermocycler (Applied Biosystems). The reaction mixtures were incubated in a 96 well plate at 95°C for 10minutes, followed by 40 cycles of 95°C for 15s and at 60°C for 1min.
For mRNA analysis, cDNA was synthesized using 500ng of RNA for each sample and the primer Script RT Reagent Kit (Takara, Japan) in accordance with the manufacturer's manual. Then, PCR was carried out with the FastStart Universal SYBR Green Master kit (Roche, Switzerland) on the ABI PRISM® 7500 Sequence Detection System (Applied Biosystems, USA) following the vendor's recommendations. The PCR temperature profile of the reaction was 95°C for 5min, 40 cycles of denaturation at 94°C for 2min and annealing and extension at 62°C for 30s, extension at 72°C for 30s.
All primers used in this study were listed in Table 1 and experiments were repeated at least three times. U6 small RNA and β-actin served as an endogenous control for normalization and quantification of miRNA and mRNA, respectively. The data were analyzed using the comparative Ct method, which was defined as 2−ΔΔCt to express the relationship for target gene expression between the experiment and control groups, where ΔΔCt=(Ct (target gene)−Ct (U6 or β-actin)) experiment group−(Ct (target gene)−Ct (U6 or β-actin)) control group.
2.4Animal grouping and model preparationMale Sprague-Dawley (SD) rats (5–6 weeks old) with a mean weight of 180±10g were purchased from Shanghai Laboratory Animal Center (SLAC, China) and housed five per cage under specific pathogen-free conditions. All animal experiments were approved by the Animal Care and Use Committee of The First People's Hospital of Kunming City and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were kept in a temperature-controlled environment (20–22°C) and 75±2% relative humidity with a 12h light/dark cycle, allowed standard chow and water ad libitum, and acclimatized for one week before the experiment. Then, liver fibrosis model was constructed and rats were randomly separated into the following six treatment groups (n=5 animals per group): Model-0 week group, Model-2 week group, Model-4 week group, Model-8 week group, Model+Negative control (NC, injected with NC plasmid) group, and Model+miR-125b (injected with miR-125b mimic) group.
Animals in Model-0 week group were not treated with CCl4 only as a control. However, the rats in Model-2-weeks group, Model-4-weeks group and Model-8-weeks group received a subcutaneous injection of 10mL/kg carbon tetrachloride (CCl4) dissolved in olive oil (25%, v/v, freshly prepared) twice a week for 2 weeks, 4 weeks and 8 weeks, respectively. In addition, in order to further investigate the role of miR-125b, Model+NC group and Model+miR-125b group were injected intraperitoneally with NC plasmid and miR-125b mimic, respectively, during the period of CCl4 treatment twice a week for 8 weeks.
At the end of the treatments, all animals were killed by CO2 inhalation to ameliorate animal suffering after the last dose of CCl4. Blood samples were immediately collected from the abdominal aorta and then centrifuged at 4000rmp for 10min at 4°C for serum preparation. A lobe of liver tissue (∼2.0×2.0×0.3cm) from each rat was removed during surgery and washed immediately with an ice-cold phosphate buffered saline (PBS) to remove blood. Half part of each liver was fixed in a 10% formalin solution for histopathological analysis, while the other part was stored at −80°C for qRT-PCR and Western blotting (WB) analysis.
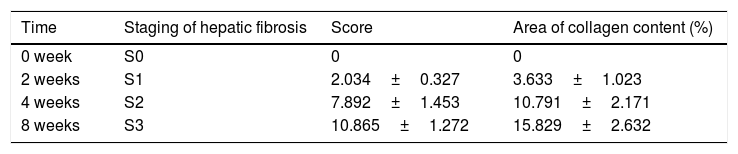
2.5Hematoxylin & eosin (HE) and Masson's trichrome stainingThe 2.0×2.0×0.3cm pieces of liver tissues were fixed in 10% formalin for at least 24h, dehydrated in graded alcohol, embedded in paraffin and cut into 4μm section. Then, sections were stained with HE staining to assess liver histology and Masson staining to assess the level of collagen deposition in five (magnification 200×) non-overlapping vision fields that is randomly selected under an Olympus microscope (Olympus IX71, Japan) and photographed in each group. The masson staining results were semi-quantified according to Ishak Scoring system [29].
2.6WB analysisThree independent samples from each group were harvested and proteins were extracted using RIPA buffer (50mM Tris–Cl, pH 8.0, 150mM NaCl, 5mM EDTA, 0.1% SDS, 1% NP-40) supplemented with protease inhibitor phenylmethanesulfonyl fluoride (PMSF) on ice for 30min. Cell lysates were centrifuged at 12,000rpm for 10min at 4°C, supernatants were saved denatured in isopycnic loading buffer for 5min at 100°C, and protein concentrations were determined by testing with a Coomassie brilliant blue protein quantitative kit (Jiancheng Bioengineering Institute, Nanjing, China). Protein extracts (30μg) were resuspended and subjected to 10–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then blotted onto polyvinylidene difluoride (PVDF) membranes (Millipore, USA) at 200mA for 1.5h by wet electrophoretic transfer. In order to prevent nonspecific background binding, the membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween-20 (TBST) for 1h at room temperature. Then, membranes were immunoblotted with primary antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000 dilution, as an internal reference; Abmart, USA), α-SMA (1:1000 dilution; Abcam, USA), Albumin (1:1500 dilution; Abcam, USA) and Gli3 (1:2000 dilution; Abcam, USA) overnight at 4°C and further incubated with secondary horseradish peroxidase-conjugated antibodies (1:12,000; Abmart, USA) at room temperature for 2h. Finally, protein bands were detected by developing the blots with the enhanced chemiluminescence Western blot detection kit (Beyotime, China). The band intensity was analyzed by Image-Pro Plus 6.0 software.
2.7Luciferase reporter assaysThree online prediction programs, TargetScan, miRanda, and PicTar, were used to identify the target relationship between miR-125b and Gli3. The sequence of segments with WT or mutant 3′-UTR region of Gli3 was synthesized and cloned into the pGL3 vector (GeneChem, China). All constructs were further verified by sequencing. Then, the 293T cells were seeded at 1×104 cell per well in 96-well plates. After 24h incubation, cells were co-transfected with pGL3-Gli3 3′UTR WT or pGL3-Gli3 3′UTR Mut, and miR-125b mimics or negative control (NC) mimics. Following cultivation for 48h, the cells were lysed using a dual luciferase reporter assay kit (Promega, USA) according to manufacturer's guidelines. Fluorescence intensity was measured using a GloMax 20/20 luminometer (Promega, USA) and firefly luciferase activity was normalized to the Renilla luciferase activity. Three independent experiments were performed in triplicate.
2.8Statistical analysisAll data were subjected to statistical analysis using the SPSS 18.0 statistical software (IBM Corporation, USA). Measurement data are expressed as the mean±SD from at least three independent experiments. Student's t-test or one-way ANOVA was applied to determine the differences. P<0.05 was considered a statistically significant difference.
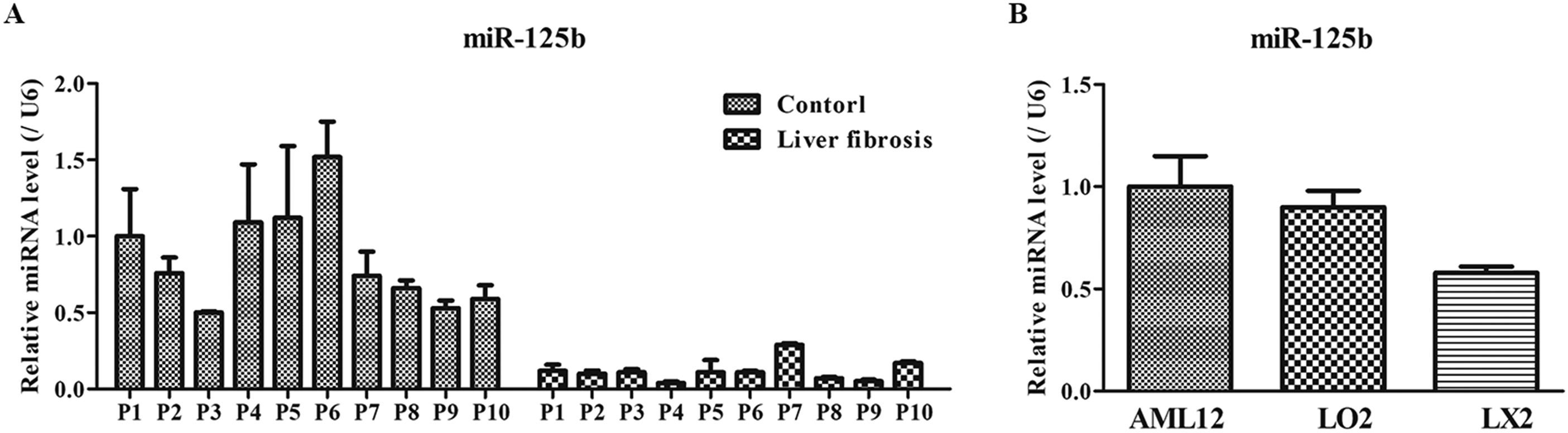
3Results3.1Expression of miR-125b in the blood specimen of liver fibrosis and liver cellsTo determine the expression of miR-125b in the blood specimen of liver fibrosis and liver cells, RT-qPCR was performed. The results showed that the miR-125b expression in healthy persons was significantly higher than that in patients with liver fibrosis (Fig. 1A). Moreover, the expressions of miR-125b in the AML12 and LO2 cells were 1.00±0.15 and 0.90±0.08, respectively, which were notably greater compared with the LX2 cells’ level (both P<0.05; Fig. 1B). Therefore, these results suggested that lower miR-125b expression might be intimately correlated with the progression of liver fibrosis.
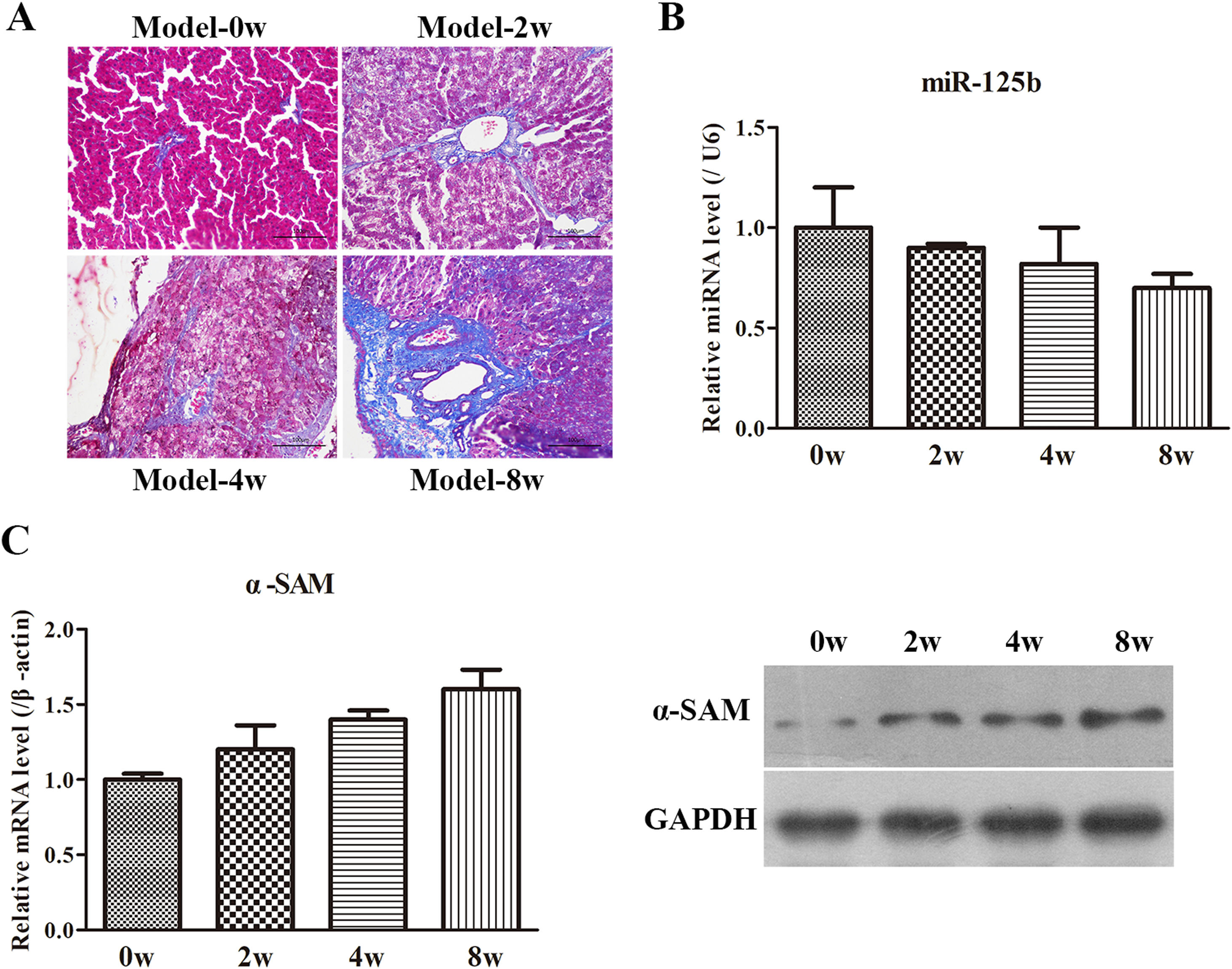
3.2Animal model observationsFirstly, the morphological changes of liver injury and fibrosis were visualized in sections by H&E and Masson's staining, scoring according to Ishak semi-quantitative scoring system. As seen in Fig. 2A and Table 2, the rats in Model-0 week group exhibited normal, clear and complete liver tissue structures with a large and round nucleus and abundant cytoplasm and with limited collagen deposition in the venous walls and the bile duct walls in the portal area. However, the rats in the other three groups displayed greater hyperplasia of fibrous connective tissue, fatty degeneration, steatosis, cell necrosis, infiltration of inflammatory cells and a larger number of collagen fibers which are mainly deposited in the portal area and interlobular septa. Moreover, the longer the molding time, the more obvious the changes are. Subsequently, the miR-125b expression was examined by RT-qPCR and the data displayed that miR-125b presented the lowest expression in Model-8-weeks group (Fig. 2B). Eventually, the expression of α-SMA, an activated HSC marker, was examined in the co-cultured system and further revealed that the α-SMA mRNA and protein levels were both gradually upregulated with the treated time (Fig. 2C). Thus, these results indicated that the rat model of liver fibrosis was successfully established.
CCl4-induced animal model evaluation. (A) The morphological changes of liver injury and fibrosis were assessed by H&E and Masson's staining, magnification 200×. (B) The miR-125b expression was determined by RT-qPCR in the CCl4-induced animal model. (C) The mRNA and protein levels of α-SMA were examined by RT-qPCR and WB, respectively.
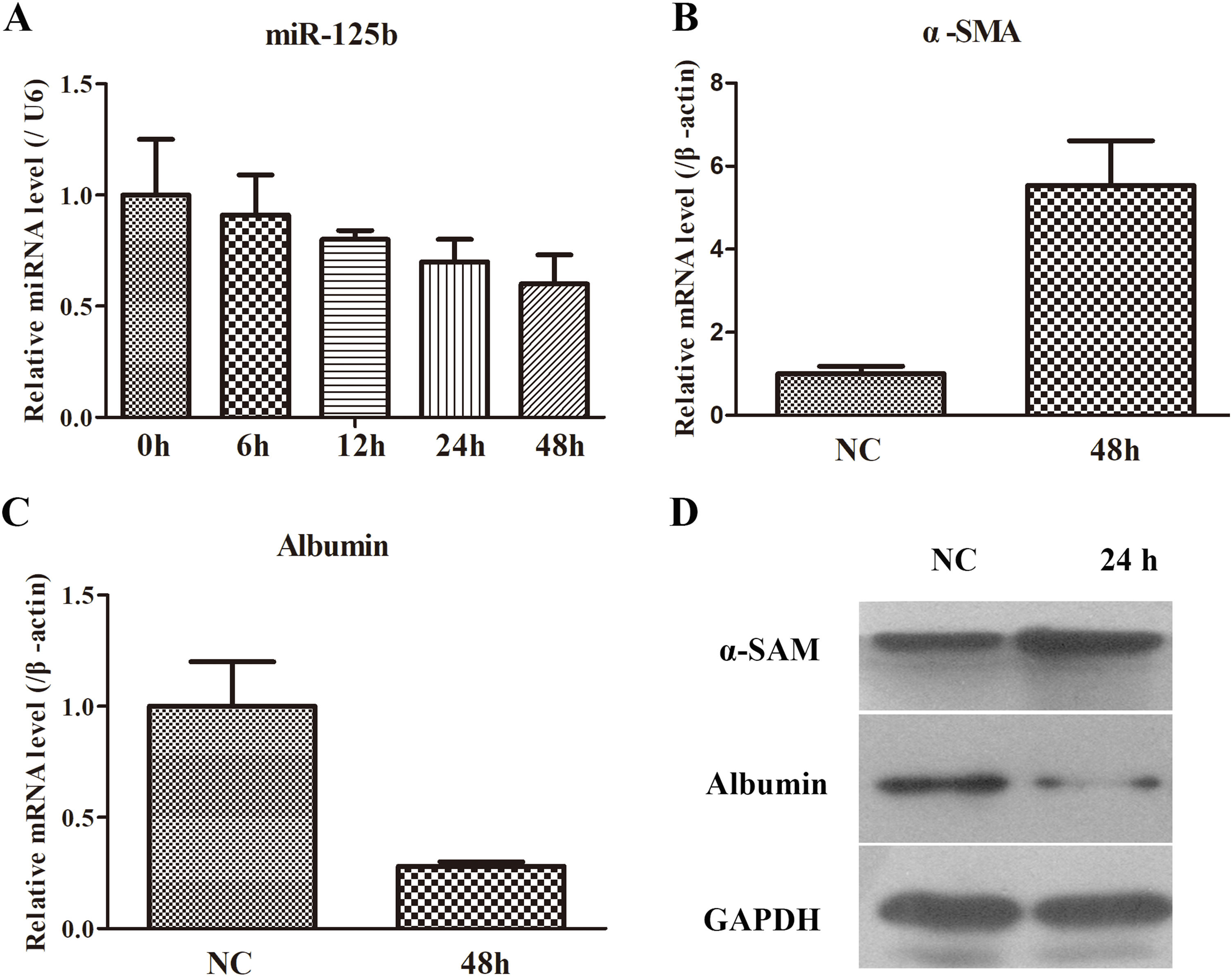
Based on the data from the animal model, the miR-125b and fibrosis-related gene expression levels were detected by RT-qPCR and WB. As exhibited in Fig. 3A, the miR-125b expression was reduced in a time-dependent manner, thereby the longest time (48h) was selected as the key time to determine the fibrosis-related genes. It was found that α-SMA was remarkably increased in mRNA and protein levels, while Albumin was markedly decreased in mRNA and protein levels (Fig. 3B–D). Hence, these data implied that the co-cultured system activated LX2 cells and miR-125b might emerge as a crucial effect in the process of liver fibrosis.
The changes of miR-125b and fibrosis-related genes in the co-cultured system of LX2 and THP-1 cells. (A) miR-125b was gradually decreased with time. (B) The mRNA expression of α-SMA was significantly up-regulated in a cellular model. (C) The mRNA expression of Albumin was notably down-regulated in a cellular model. (D) WB experiment was used to identify the protein expression of α-SMA and Albumin.
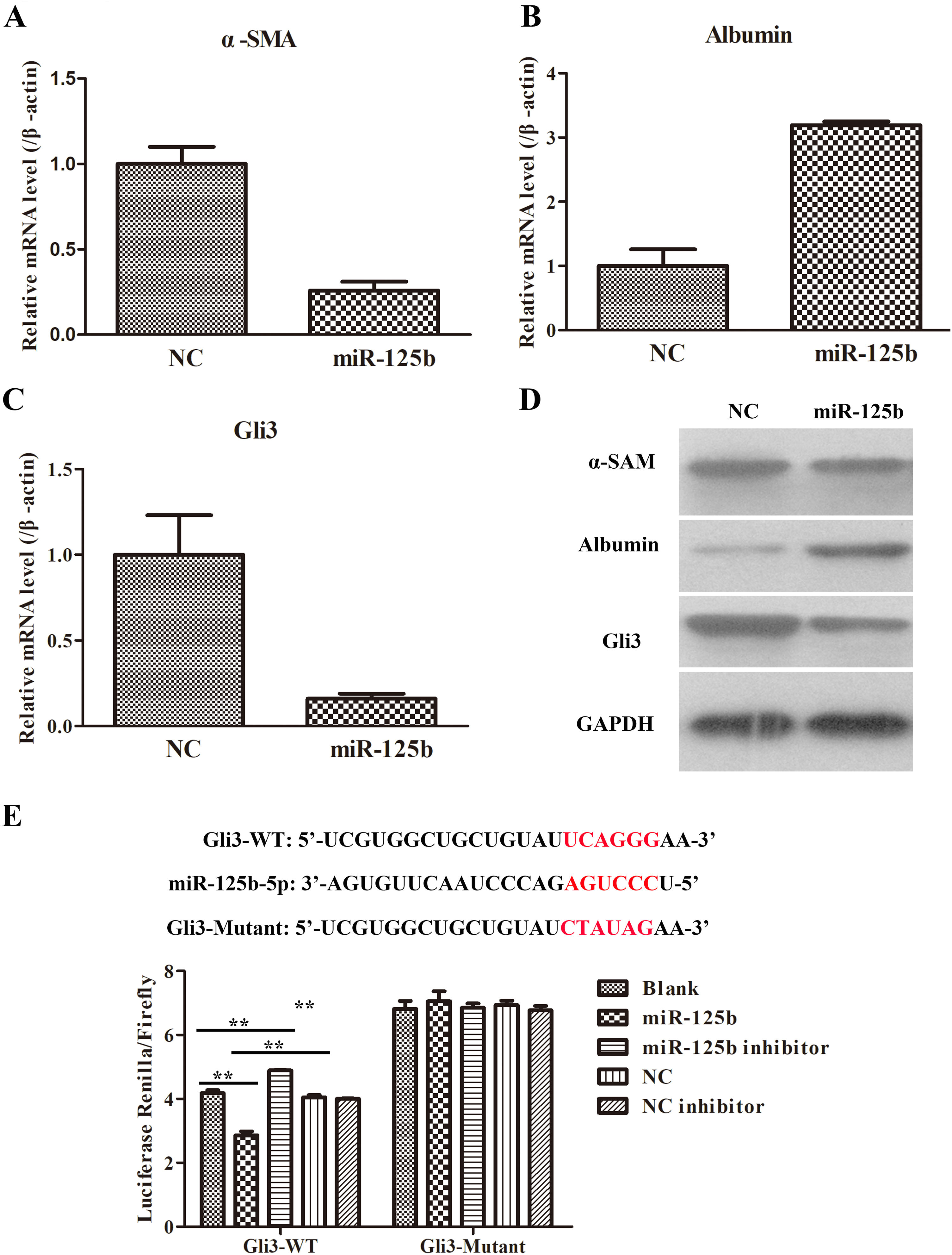
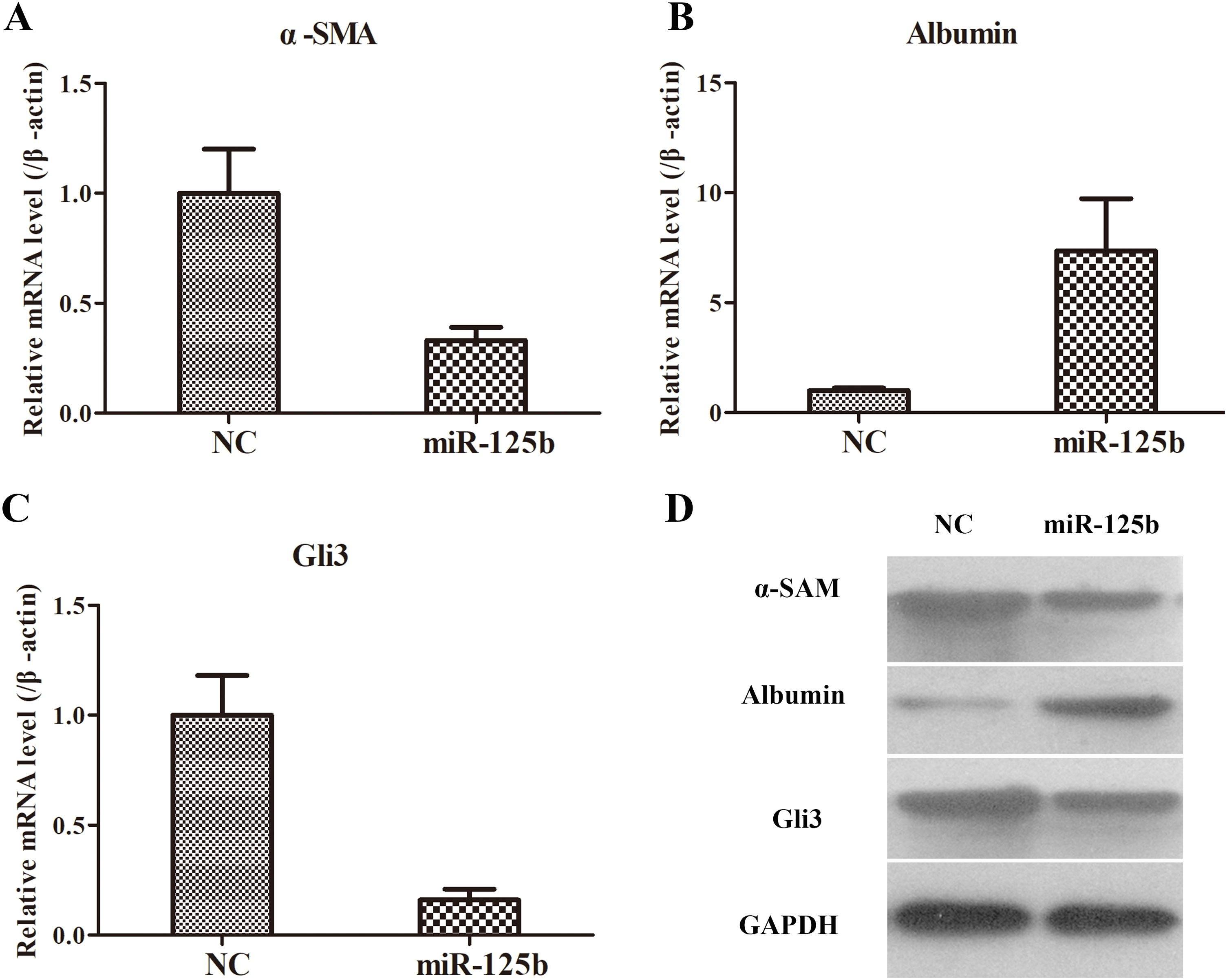
In order to investigate the effects of miR-125b on the co-cultured system of LX2 and THP-1 cells, we transfected the miR-125b mimic and NC into the co-culture system. It was discovered that the fibrosis-related genes, such as α-SMA and Albumin, were obviously down-regulated and up-regulated, respectively, in miR-125b-overexpression group (Fig. 4A and B). Furthermore, the protein expressions of α-SMA and Albumin were also similar to their corresponding mRNA levels in miR-125b-overexpression group (Fig. 4D). Additionally, the potentially supposed target (namely, Gli3) of miR-125b was conspicuously degraded in miR-125b-overexpression group as compared with the NC group (Fig. 4C and D). In consequence, these data concluded that miR-125b could decelerate α-SAM and Gli3 expressions, and accelerate Albumin expression.
The influences of miR-125b in activated LX2 cells. (A) Overexpression of miR-125b. inhibited the α-SMA expression in mRNA level. (B) Enhanced miR-125b promoted Albumin expression in mRNA level. (C) Up-regulation of miR-125b suppressed Gli3 expression in mRNA level. (D) The effects of miR-125b on the protein expressions of fibrosis-related genes, including α-SMA, Albumin, and Gli3. (E) Dual-luciferase assays were applied to verify the target relationship between miR-125b and Gli3.
Bioinformatic analysis has predicted the potential target interaction of miR-125b and Gli3. Moreover, the above results have also demonstrated that there was an inverse expression trend between miR-125b and Gli3. So, to further verify whether miR-125b directly acts on the 3′-UTRs of Gli3, dual-luciferase reporter assays were carried out using 293T cells. As illustrated in Fig. 4E, the miR-125b mimics significantly decreased the luciferase activity of the Gli3 3′-UTR-dependent reporter, but it did not affect the luciferase activity of the mutant reporter. In addition, the mimics control had no effect on either WT or mutant reporter luciferase activity. Collectively, these results uncovered that miR-125b might directly act on the 3′-UTRs of Gli3 mRNA.
3.6The roles of miR-125b in rat model of liver fibrosisAfter demonstrating the effects of miR-125b in the co-cultured system of LX2 and THP-1 cells, we eventually explored the roles of miR-125b in a rat model of liver fibrosis. Similarly, the fibrosis-related genes, including α-SAM, Albumin, and Gli3, were the essential indexes. The changed trends of α-SAM, Albumin, and Gli3 both in mRNA and protein levels were surprisingly coincident to the expression pattern in cellular levels (Fig. 5). Taken together, these data pointed out that miR-125b could suppress α-SAM and Gli3 expressions and promote Albumin expression in in vivo and in vitro settings.
The roles of miR-125b in an animal model. (A) The α-SMA expression in mRNA level was reduced after giving miR-125b mimic in liver tissues of CCl4-treated rats. (B) The Albumin expression in mRNA level was elevated when administrating miR-125b mimic in liver tissues of CCl4-treated rats. (C) The Gli3 expression in mRNA level was declined following miR-125b treatment in liver tissues of CCl4-treated rats. (D) The effects of miR-125b on the protein expressions of fibrosis-related genes, including α-SMA, Albumin, and Gli3, as examined by WB.
Hepatic fibrosis results from an excessive scarring response to chronic liver damage in accompany with the accumulation of ECM proteins, mainly type I fibrillar collagen (Collagen I) and finally may progress to liver cirrhosis, liver cancer and liver failure [1]. Recent reports have shown that miRNAs, stable in circulation systems, and tissue or organ-specific cells, can often be detected under pathological conditions, thereby altered miRNA levels are also served as biomarkers in various diseases [12]. Moreover, it has been revealed that aberrant miRNAs are intimately associated with the HSC activation and liver fibrosis [14,16]. However, the underlying mechanisms have not been clearly elucidated. Previous studies demonstrated that miR-125b was downregulated in HCC tissue, and plasma miR-125b may be a diagnostic marker for HCC [30]. Furthermore, miR-125b suppressed metastasis of hepatocellular carcinoma by disrupting the formation of vessels that encapsulate tumor clusters [31]. Additionally, miR-125 played a crucial role in HBV replication and HBV-associated disease [23]. Therefore, we first examined the expression of miR-125b in serum samples of clinical patient, activated LX2 cells and the liver tissues of CCl4-induced rat model. It was discovered that miR-125b was significantly declined in the above specimen, which suggested that miR-125b might exert a pivotal role in the progression of liver fibrosis.
Then, the fibrogenesis-related indexes, including collagen deposition and α-SMA expression, in in vivo and in vitro model were detected. Generally, once HSCs were activated, HSCs became proliferative and induced α-SMA or Collagen I expressions [16]. Thereby, α-SMA or Collagen I was considered as the markers of fibrogenic cell activation. However, our result showed that collagen deposition and α-SMA expression were both notably increased in in vivo and in vitro. In addition, the Albumin was markedly decreased in mRNA and protein levels in a cellular model, while Albumin and Gil3 expressions were remarkably increased and decreased, respectively, after giving miR-125b plasmid. Hh signaling, mainly involved in adult wound healing, is initiated by the interaction of Hh receptor Patched (Ptc), Hh ligands (Sonic hedgehog-Shh, Indian hedgehog-Ihh, and Desert hedgehog-Dhh), and other molecules (e.g., Gli3) [25]. Hence, the Gli family, especially Gli3, is a better target for biomarker and therapeutic approach aimed at controlling activation of HSCs during liver fibrogenesis [25,26]. Our results implied that miR-125b could negatively regulate Gli3 and implicate in the progression of liver fibrosis. Moreover, dual-luciferase assays further manifested that miR-125b could directly target the 3′-UTR of Gli3.
Ultimately, in order to uncover the effects of miR-125b and Gli3, we tested the expression of fibrogenesis-related genes, such as α-SMA, Albumin, and Gil3. After giving miR-125b mimic to CCl4-treated rats, the expressed patterns of α-SMA, Albumin, and Gli3 in liver tissues of CCl4-treated rats were a coincidence with those in the activated LX2 cells, implying that elevated levels of miR-125b ameliorate the damages of liver fibrosis by directly regulating Gli3.
Previous studies have shown that some miRNAs, such as miR-9, miR-214 and miR-378, etc., play important roles in liver fibrosis. In this study, we identified a new miRNA, miR-125b, that inhibits liver fibrosis. It has become very important to study the interactions between miR-125b and the above-mentioned liver fibrosis-related miRNAs, which will help us better understand the underlying mechanism of liver fibrosis. Interestingly, a very recent study showed that miR-125b promoted hepatic stellate cell activation and liver fibrosis by activating RhoA signaling [32], which seems to contradict our findings. This may be explained by the idea that miR-125b may play different or even opposite functions by acting on different target genes or signaling pathways, and further research is needed to explore this.
Taken together, in our study, we have confirmed that miR-125 was reduced with the development of liver fibrosis. We also verified the target interaction between miR-125 and Gli3. In addition, overexpression of miR-125b by administrating miR-125b mimic in CCl4-induced liver fibrosis rats and activated LX2 cells could decelerate pro-fibrotic gene expression and accelerate anti-fibrotic gene expression. So, these findings concluded that miR-125b and its target might serve as a novel regulator in the pathogenesis of liver fibrosis and provide useful insights into the underlying mechanisms of liver fibrosis.
List of abbreviationsHBV hepatitis B extracellular matrix hepatic stellate cells GLI family zinc finger 3 hepatocellular carcinoma carbon tetrachloride phenylmethanesulfonyl fluoride
The authors declare that there was no conflict of interest in this study.
This study is supported by The Joint Special Project Sponsored by Science and Technology Department of Yunnan Province and Kunming Medical University, Postdoctoral Fellow Special Project of The First People's Hospital of Kunming City, The Foundation for Outstanding Young Scientist in Kunming City, The Ten-Hundred-Thousand Project and The Foundation for Clinical Experts Sponsored by Kunming Municipal Health and Planning Commission, Yunnan Province Applied Basic Research (No. 2017FE468(-099)), and Health Technology Project of Yunnan Province (2018NS0162 and 2017NS080).