Trastuzumab is a monoclonal antibody targeted against the Human Epidermal Growth Factor Receptor 2 (HER2) overexpressed in some breast cancer. This targeted therapy significantly improves the prognosis of these cancers. Recently an anti-HER2 antibodydrug conjugate was shaped in order to facilitate the targeted delivery of potent cytotoxic drug to cancer cells and to reduce resistance. This formulation, called trastuzumab emtansine (T-DM1), consists of the monoclonal antibody trastuzumab linked to a cytotoxic drug (a derivative of maytansine) via a chemical linker. Little is known about adverse reactions due to this new formulation. Herein we described the case of a woman suffering from a HER2-positive breast cancer, treated with trastuzumab for 30 months followed by T-DM1 monotherapy. After 12 months of T-DM1 treatment, a nodular regenerative hyperplasia confirmed by liver biopsy occurred. T-DM1 was stopped and medical imagery showed a resolution of the nodular regenerative hyperplasia. Unfortunately, hepatic metastasis progressed. Few cases of nodular regenerative hyperplasia induced by T-DM1 have been described so far. Further studies are needed to explore pathogenesis of nodular regenerative hyperplasia with this new antibody-drug conjugate treatment.
Approximately 20% of breast cancers overexpress Human Epidermal Growth Factor Receptor 2 (HER2). Due to this overexpression, those are more aggressive and patients have a significantly shortened disease-free survival and overall survival than in cancer without HER2 overexpression.1,2 Trastuzumab is a monoclonal antibody directed against the extracellular domain of HER2 and one of the first active HER2-directed therapy. Trastuzumab improved meaningfully survival of women suffering from positive breast cancer,3–5 even in case of metastases.6 Resistance to trastuzumab has been studied.7,8 Lately, another formulation of trastuzumab was shaped, called trastuzumab-emtansine (T-DM1), based on an antibody-drug conjugate composed of trastuzumab, a thioether linker (SMCC) and DM1, a derivative of the antimicrotubule agent maytansine. Emtansine refers to DM1. Once bind to HER2, T-DM1 is internalized and undergoes proteolytic digestion, releasing the cytotoxic microtubule inhibitor within the target cells.9 Thanks to the microtubule inhibitor emtansine, T-DM1 extends the trastuzumab-mediated effects in blocking HER2-mediated signal transduction and facilitates antibody-dependent cell-mediated cytotoxicity.10,11
Thrombocytopenia is one of the most common adverse reaction (ADR) with T-DM1 (grade 3 in 14%, grade 4 in 3%, nadir by day 8).12 Nausea is also frequent, concerning 40% of exposed patients. Hepatotoxicity of T-DM1 has been described13 with mostly increased liver aminotransferases. Herein we report a case of T-DM1 monotherapy induced nodular regenerative hyperplasia (NRH), after 12 months of treatment.
Case ReportA 48 year-old woman was diagnosed with an invasive ductal carcinoma hormone SBR I receptor-positive HER2-negative in 2008, initially treated by surgery and cancer chemotherapy with docetaxel, doxorubicin, and cyclophosphamide for 6 cycles. In July 2012, cancer recurrence occurred, with bone, nodal and mediastinal metastases. Biopsy showed an overexpression of HER2 status. Second-line chemotherapy was initiated with weekly paclitaxel and trastuzumab 6 mg/kg every 3 weeks for 6 months followed by a trastuzumab monotherapy. In January 2015, due to cancer progression, trastuzumab was stopped and switched to T-DM1 3.6 mg/kg every 3 weeks. From January to December 2015, 16 T-DM1 courses were performed. Before initiating T-DM1, liver function tests were normal.
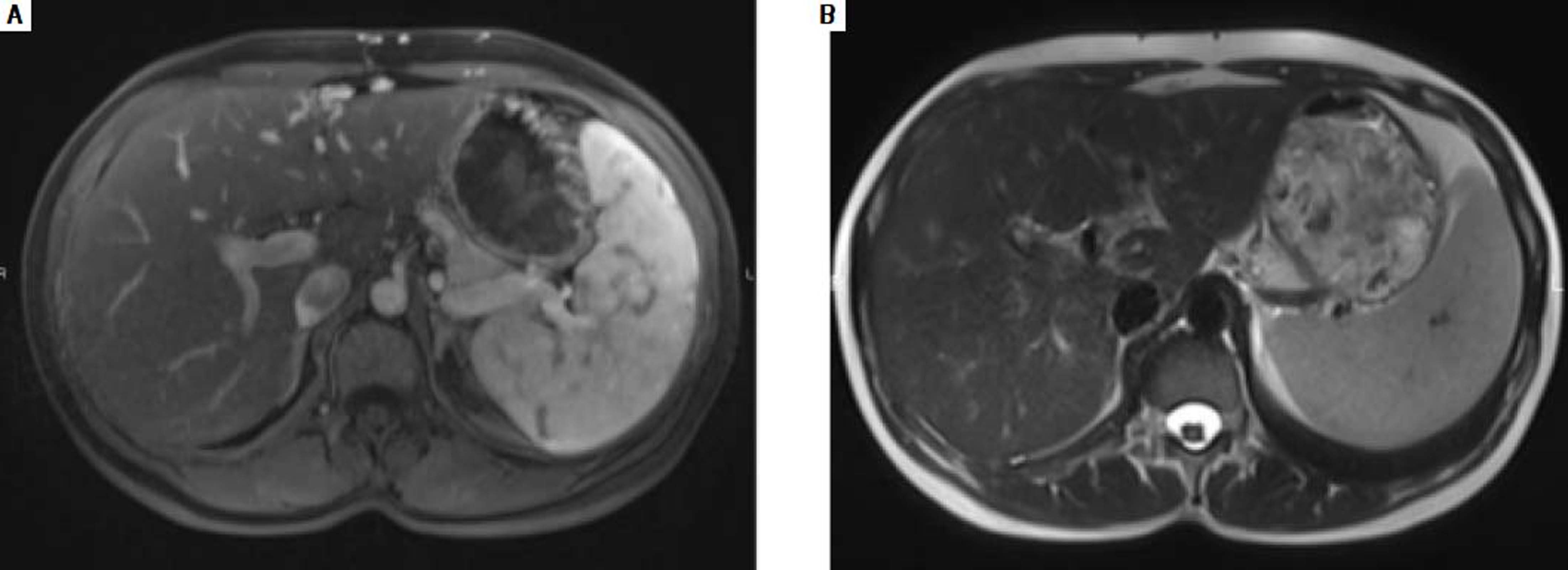
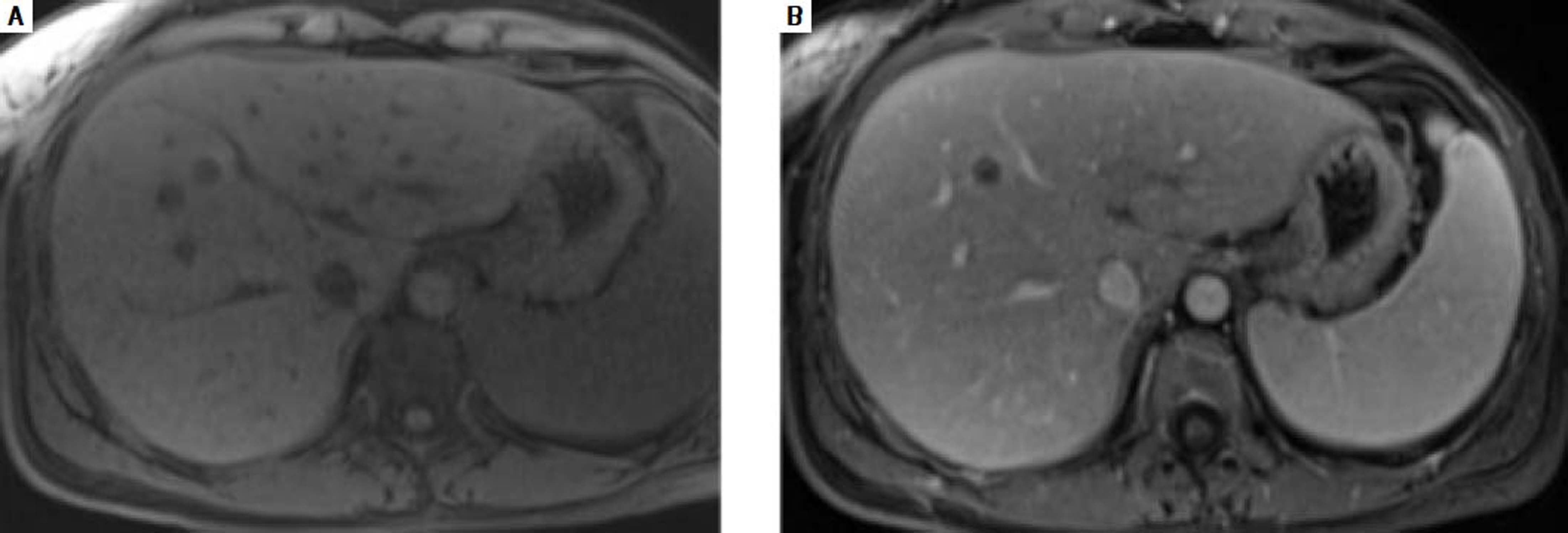
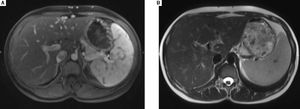
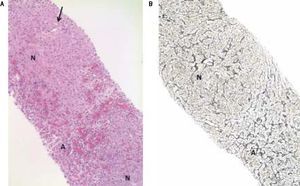
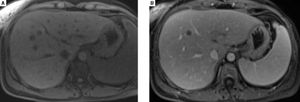
In December 2015, clinical examination showed a hepatosplenomegaly with mildly disturbed liver function tests: increase of transaminases was less than to 2fold the upper limit of normal). In January 2016, MRI revealed a mild hepatic dystrophy with recanalization of para-umbilical vein for signs of portal hypertension, homogenous splenomegaly (Figure 1). There was neither ascites cirrhosis nor metastases. T-DM1 was immediately stopped in January and trastuzumab alone was started again. In February 2016, histologic examination of liver biopsies showed evidence of vascular sinusoidal pathology compatible with NRH (Figure 2). Other causes of NRH were excluded, especially portal vein metastasis. Hepatitis serologies were negatives. Serum alpha-fetoprotein was negative and there was no argument for an autoimmune pathology. In March 2016, MRI showed hypodense nodules (Figure 3), suggestive of liver metastases, confirmed further by the biopsy. Due to the cancer progression, trastuzumab was definitely stopped and the patient underwent multiple chemotherapy regimens, including capecitabine and then vinorelbine. In May 2016, all liver function tests returned in normal ranges. Unfortunately, one month later, liver metastases dramatically progressed, leading to switching again to paclitaxel.
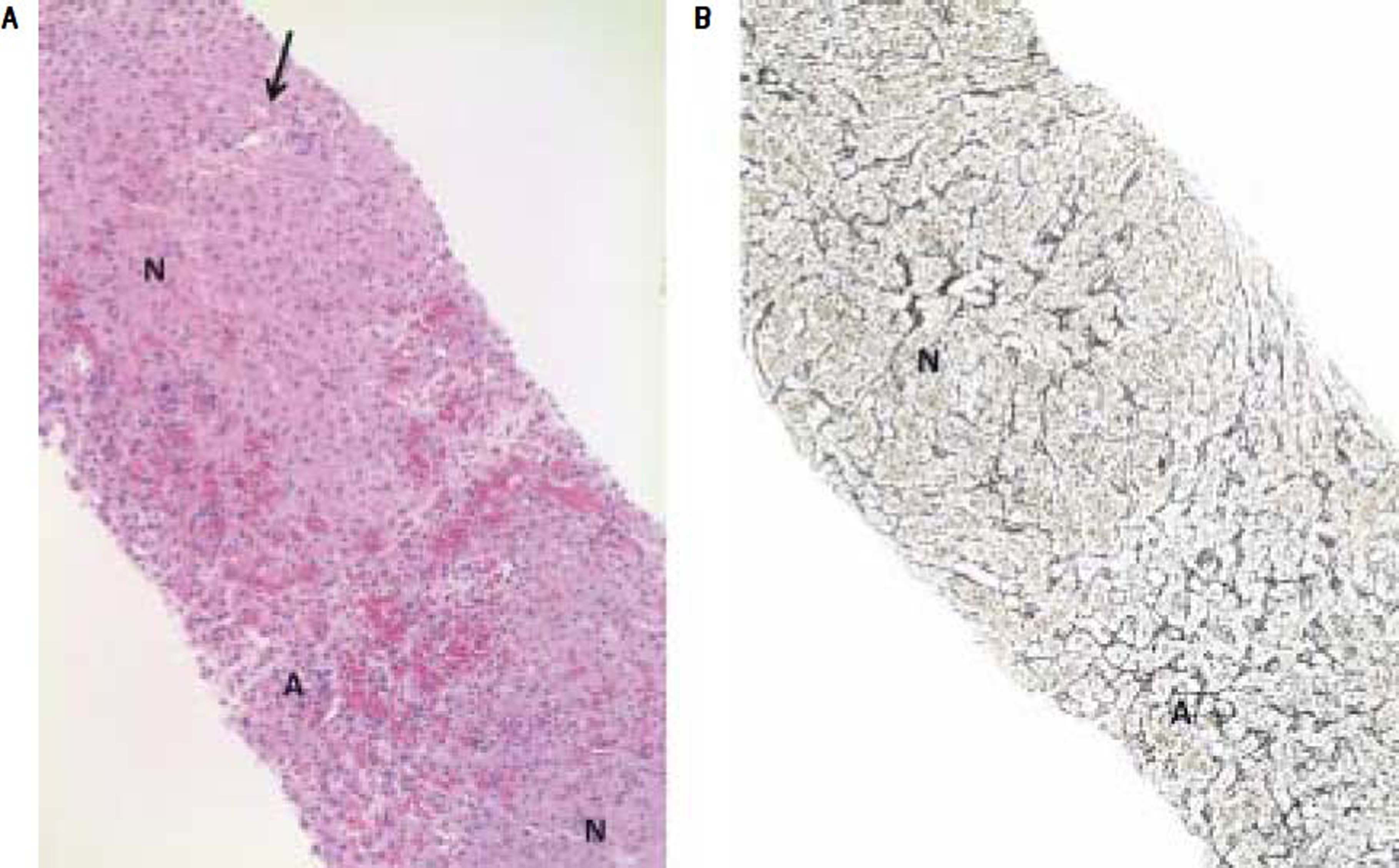
A. Needle liver biopsy specimen showing abnormal parenchymal pattern with alternating nodular foci (N) without cirrhosis and atrophic foci (A) with blood-filled spaces at the place of thin liver cell plates and altered sinusoids. Note the non fibrotic portal tract (arrow) in the center of the upper nodule (HES, objective × 10). B. Hyperplastic parenchymal nodule (N) with thickened liver cell plates perpendicularly arranged to the atrophic hepatocellular foci (A) (Reticulin, objective × 10).
NRH is characterized by multiple hyperplastic liver nodules without cirrhosis. This phenomenon is the consequence of heterogeneous hepatic perfusion.14–16 On the one hand, the portal flow is decreased, due to portal vein occlusion, leading to an ischemia and an atrophy of the liver cell plates. On the other hand, vascularization increases to compensate the portal flow and lead to nodular hyperplasia of unaffected adjacent hepatocytes leading to NRH.17 This pathology is frequently associated with systemic or malignant diseases.
Drug-induced NRH is rare and typically occurs after months or years of treatment except for oxaliplatin. Most common drugs involved in NRH are thiopurines, antiretroviral agents, and platinum salts. NRH development is asymptomatic at early phase with mild increase of the transaminases. Significant portal hypertension and its complications, especially hypersplenism, ascites, variceal bleeding, appear later. Mechanism of drug-induced NRH is not fully understood. Some drugs could induce venous and sinusoidal injuries, resulting in parenchymal changes.17 In the same way, drug-induced vasculitis could promote portal obstruction.
Five case of T-DM1 induced NRH has been reported so far in the literature. Three cases were biopsy-confirmed in clinical trials, at different courses: cycle 3, 20 and 37. One patient worsened to liver failure and died.18 Recently, Force, et al. described two other cases of NRH occurring after 15 and 17 months of T-DM1 treatment combined with other drugs, pertuzumab and oral GDC-0941 (clinical trial).19 After drug withdrawal, regression of portal hypertension was described.
In our case, the patient treated with T-DM1 monotherapy for 12 months before early stage NRH diagnosis. Liver function tests resolved within 5 months after withdrawal, which is consistent to previously reported cases.
According to the World Health Organization adverse drug reaction (ADR) database, 19 cases of NRH were attributed to T-DM1. The Information Component, which measures the disproportionality between the observed and the expected reporting of a drug-ADR pair, is 4.49. This positive value indicates that T-DM1 induced-NRH is reported more often than expected, based on all the reports in the database. In comparison, 6 cases of NRH were reported with trastuzumab and the Information Component is 1.75. Though, it is interesting to notice that T-DM1 was also considered as a causative drug of NRH for 4 of these 6 cases.
Clinical studies with T-DM1 showed frequent increase of hepatic aminotransferases: 23.5% for serum AST and 15.7% for serum ALT, mainly grade 1 or 2.18 To investigate T-DM1-induced hepatotoxicity, Yan et al. used hepatocytes and mouse models.13 They revealed that T-DM1 was internalized into human hepatocytes, once bind to HER2. After endocytosis, T-DM1 induced apoptosis and inhibited hepatocytes’ growth by mitotic arrest. Moreover, in mouse liver, T-DM1 increased serum hepatic aminotransferases, lactate dehydrogenase, and Tumor Necrosis Factor alpha in a dose-dependent manner, compared to trastuzumab and placebo. Necrosis and inflammation were also highlighted in mouse liver tissue after T-DM1 treatment, whereas trastuzumab induced only inflammation and no necrosis.
According to World Health Organization ADR database and previous data,13 T-DM1 seems to be associated with more frequent hepatotoxicity than trastuzumab. This could be explained by its antibody-drug conjugate structure. A former antibody-drug conjugate, gemtuzumab ozogamicin, was withdrawn from the market for safety reasons and lack of efficacy. Gemtuzumab ozogamicin was composed of gemtuzumab, an anti-CD33 monoclonal antibody, linked with calicheamicin. This drug was indicated for the treatment of acute myeloid leukemia (AML).20 Calicheamicin is an antitumor antibiotic which binds to DNA, after internalization in the AML cell, causing breaks and cell death. More than 100 cases of sinusoidal obstructive syndrome occurred during gemtuzumab ozogamicin treatment in clinical trials.21 Liver biopsies of 23 patients after gemtuzumab ozogamicin infusion revealed hepatocyte necrosis as a consequence of the sinusoidal injury leading to an ischemia more than a direct toxic effect of calicheamicin on hepatocytes.23
According to the pharmacokinetic study, the major component in rat plasma was the intact antibody-drug conjugate T-DM1. No accumulation of T-DM1 nor catabolites was found.24 Plasma concentrations of emtansine, and thiolinker SMCC-emtansine were low. These data were confirmed in human plasma, suggesting low systemic exposure of these components. Following endocytose, T-DM1 is catabolized by lysosomes into trastuzumab and emtansine-SMCC. The antibody part, trastuzumab, is degraded into peptides or amino acids without biological activity. After cleavage from the thiolinker, the microtubule inhibitor DM1, a small molecule, is metabolized by CYP3A4 and 3A5 into less active metabolites. The major catabolites in rat and human were DM1, emtansine and lysine-emtansine.24 They are mainly eliminated in bile and feces (> 80%).
The conjugate of the antibody-drug is generally a highly cytotoxic agent because very little of the administered conjugate reaches its targeted tumor cells in vivo.25 DM1 is a maytansinoid, ie a derivative of maytansin, an antimitotic agent much more cytotoxic than conventional cancer chemotherapeutic agents such as vincristine.25 About 3.5 molecules of DM1 are linked per antibody through the thioether linker SMCC.10,26 The DM1 catabolites were less cytotoxic toward human carcinoma cell lines than maytansine.25,27 This information suggested the important role of the liver in detoxification of emtansine. Other microtubules inhibitors such as colchicine or vinca alkaloids are not implicated in the development of NRH in treated patients. Previous studies revealed that maytansine can induce thrombocytopenia, nausea, diarrhea28 but no hepatotoxicity has been described so far.
Effects of the thioether linker SMCC on liver are unknown, just as its way of detoxification. In preclinical rodent models, this linker was better tolerated with T-DM1 than others.29,30 Linker had an impact on the clearance of T-DM1 which was longer than other linkers, due to its non-cleavable properties.26
Detoxification during hepatobiliary elimination of DM1 and thiolinker SMCC might play a role in the hepatotoxicity of T-DM1 compared to trastuzumab. Even if DM1 oxidized metabolites showed less cytotoxicity, they could participate in liver injury, including NRH. The relationship between thiolinker SMCC and HNR development is not ruled out too. DM1 and SMCC could also interact with hepatic endothelial cells.
This case-report showed that T-DM1 monotherapy can induce NRH. The role of T-DM1 is stronger in this case report as it is the only drug. Data from World Health Organization ADR database supports this finding. Further studies are needed to explore pathogenesis of NRH during T-DM1 treatment.
Abbreviations- •
ADR: adverse drug reaction.
- •
HER2: Human Epidermal Growth Factor Receptor 2.
- •
NRH: nodular regenerative hyperplasia.
- •
T-DM1: trastuzumab emtansine.
The authors declares that there is no conflict of interest regarding the publication of this case report.