The prevalence of under nutrition in cirrhotic patients is 61% and it usually progresses as the disease becomes more advanced. The deterioration in the nutritional status and its associated metabolic derangements has raised doubts about the benefits of severe and prolonged protein restriction as a treatment for hepatic encephalopathy. However, the practice of dietary protein restriction for patients with liver cirrhosis is deeply embedded among medical practitioners and dietitians. To date, no solid conclusions may be drawn about the benefit of protein restriction. However, the negative effects of protein restriction are clear, that is, increased protein catabolism, the release of amino acids from the muscle, and possible worsening of hepatic encephalopathy. In conclusion, chronic protein restriction causes progressive and harmful protein depletion and must be avoided.
Among the multiple functions of the liver, protein metabolism is a mainstay for life support, as the liver synthesizes most of the body proteins from amino acids absorbed from the digestive tract. Protein degradation is also important, as it generates free amino acids, which may be transaminated or deaminated, producing ammonia. This toxic metabolite is effectively removed from the circulation by hepatic conversion to urea, which is then eliminated in the urine by the kidney. However, when liver function is impaired, hyperammonemia ensues, increasing the likelihood of developing hepatic encephalopathy.
Undernutrition –particularly protein-calorie malnutrition– is highly prevalent in patients with liver cirrhosis; nevertheless, its occurrence varies widely, depending on the selection of nutritional assessment parameters. A recent study estimated that the prevalence of undernutrition in cirrhotic patients is 61%,1 and although it may not be related to the cause of liver disease, it usually progresses as the disease becomes more advanced,2 reflecting the severity of the disease. A prospective study on a large series of cirrhotic patients showed that severe malnutrition –including depletion of lean body mass– is an independent prognostic factor for the survival of patients with liver cirrhosis.3
Liver cirrhosis increases nutritional requirements and clearly increases morbidity and mortality. Undernutrition may be explained by metabolic derangements that accompany liver damage and by hypermetabolism, hypercatabolism, malabsorption and decreased ingestion because of anorexia, early satiety and nausea/vomiting. The lack of accurate data on the effect of nutrition on the outcome of liver disease and on the possible benefits of not restricting dietary protein intake (vide infra) may aggravate protein-calorie undernutrition in these patients.
General AspectsPathophysiology of hepatic encephalopathy is yet the matter of discussion, and several theories have been proposed. Among them, the ammonia theory is most widely spread and best supported;4 other theories implicate the ratio of branched-chain amino acids (BCAAs: Ile, Leu, Val) vs. aromatic amino acids (AAA; Phe, Tyr, Try), GABA, false neurotransmitters, serotonin, mercaptans, phenols, short-chain fatty acids and, most recently, manganese.
Ammonia may originate from dietary proteins or the activity of intestinal urease or intestinal or renal glutaminase. Surprisingly, nearly 85% of total blood ammonia may be generated by intestinal glutamine deamination, whereas as little as 10-15% may originate from the deamination of proteins by the gut macrobiota.5 Ammonia is undoubtedly toxic, and must be removed from the bloodstream by hepatic conversion to urea and elimination of urea by the kidney, or even as nonconverted ammonia in urine. The importance of muscle glutamine formation as a means of removing ammonia from the bloodstream has also been stressed recently, implying that skeletal muscle plays a crucial role in ammonia detoxification. However, the role of muscle glutamine formation in ammonia detoxification is mitigated to an extent by the reconversion of glutamine to glutamic acid and ammonia in the gut.6 Nonetheless, the liver and muscle play central roles in ammonia detoxification by converting it to urea (liver) and glutamine (liver and muscle).5
Insulin resistance is a frequent finding in advanced liver disease.7 The inability of the diseased liver to produce glucose via glycogenolysis increases the utilization of alanine and glycerol, causing catabolism of muscle and adipose tissue, respectively. Whereas the BCAA-to-AAA ratio is 3:1 or 4:1 in healthy individuals, patients with liver cirrhosis exhibit a ratio of 1:1,8 possibly because muscle stores BCAAs or because BCAAs are used by the kidneys as substrates for gluconeogenesis during insulin resistance.9
During the past decade, elucidation of the progressive deterioration in the nutritional status of patients with liver cirrhosis and its associated metabolic derangements has raised doubts about the benefits of severe and prolonged protein restriction as a treatment for hepatic encephalopathy10 because of a lack of scientific proof.11 Swart, et al. showed that a protein-restricted diet supplying 40 g of protein per day was unable to achieve positive nitrogen balance in cirrhotic patients and suggested that the protein requirement of these patients is elevated.12 Studies on malnourished cirrhotic patients reported that they retained nitrogen upon repletion feeding at a rate greater than that considered normal, similar to that observed with repletion feeding of underweight, healthy individuals,13 and that protein repletion feeding induces a significant increase in protein synthesis.14 Therefore, chronic protein restriction, which increases protein catabolism and the release of amino acids from the muscle, causing progressive and harmful protein depletion,15 must be avoided.16-19
Protein restriction was first proposed on a theoretical basis20-22 and according to anecdotal cases, but as recently as 1989, Sherlock recommended as little as 20 g of protein per day23 as a therapy for hepatic encephalopathy. However, since these reports were published, the only randomized study conducted found no difference in the development of hepatic encephalopathy between a protein-restricted diet and a normal diet.19 Although several meta-analyses have been performed, no solid conclusions could be drawn about the benefit of protein restriction because of great variation in study design and the considerable number of confounding factors that were not properly controlled for in the studies. However, the negative effects of protein restriction are clear, that is, increased protein degradation and possible worsening of hepatic encephalopathy,6 worsening of nutritional status and increased mortality because of alcoholic liver disease. Therefore, dietary protein restriction cannot be recommended at present. Nonetheless, the practice of dietary protein restriction for patients with liver cirrhosis is so deeply embedded among medical practitioners and dietitians15 that it may be years before it is abandoned.
Patients with liver disease may differ in their tolerance to protein, depending on the dietary amino acid profile and fiber content. Vegetable proteins are thought to be best tolerated, followed by proteins contained in dairy foods –mainly milk– which may also contain lactose, a disaccharide that exerts a similar effect in lactose-intolerant people to that exerted by other nonabsorbable disaccharides used in the treatment of hepatic encephalopathy, such as lactulose and lactitol.
Vegetables are thought to be beneficial not only because of their high content of fiber, which promotes bacterial fermentation and decreases colonic transit time, decreasing ammonia absorption from the gut, but also because of their high BCAA content, low Met and Try contents, and the induction of gut microbiota which, in turn, increases fecal nitrogen excretion.15 The effectiveness of a vegetarian diet was proven by Bianchi24 and Uribe;25 however, no positive effects were shown by Shaw26 or Chiarino.27 Nevertheless, also supporting the underlying rationale for the use of vegetable proteins is the fact that dietary fiber contributes to the improvement of glycemic control in these patients.28 However, as a diet containing more than 40% vegetable protein causes bloating, flatulence and early satiety, vegetarian diets are frequently poorly tolerated in the long term. To obtain a more palatable and varied dietary regimen, dairy foods may be added; this high-protein diet is well tolerated and has proven to be beneficial in patients with cirrhosis and hepatic encephalopathy.29
The primary goal of the treatment of hepatic encephalopathy is a reduction in blood ammonia level, which can be achieved by supplementation with AACR, ketoanalogues, L-ornithine L-aspartate (or its ketoanalogues) or zinc (an important coenzyme in the urea cycle). In 1973, Rudman measured the ammonia content of several food items and proposed that the avoidance of food with high ammonia content (blood, gelatin, brewer’s yeast, bacon) could reduce the risk of encephalopathy.30
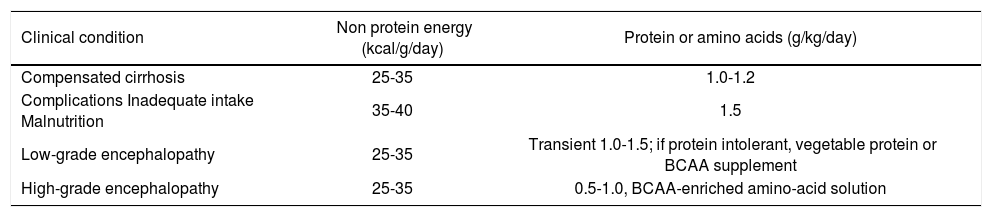
In 1997, the European Society of Parenteral and Enteral Nutrition (ESPEN) published a consensus on specific guidelines for nutrition in liver disease and transplantation.31 These guidelines increased the protein requirements of cirrhotic patients and recommended a diet containing at least 1.2 g of protein per kg body weight per day. Interestingly, they also state that hepatic encephalopathy should not be a reason to limit the protein content of the diet to 1-1.5 g of protein per kg body weight per day unless the protein restriction is very transient and is applied in conjunction with BCAA-enriched amino acid solutions. Patients with liver disease should receive sufficient energy (35-40 Kcal/kg/d) to prevent the degradation of endogenous protein to provide energy, and up to 1.6 of protein per kg body weight per day should be supplied.31,32 For patients with compensated liver cirrhosis, this goal can be achieved with a normal diet without restricting its carbohydrate, protein or fat content. In cases of uncompensated cirrhosis, supplementary BCAAs are often recommended and prescribed.33 According to the ESPEN consensus report of 2006,31 low-grade hepatic encephalopathy (grades I and II) is not regarded as a reason for dietary or protein restriction, indicating that malnutrition is certainly considered a negative prognostic factor. However, patients with severe hepatic encephalopathy (grades III and IV) are exceptions, and should be deprived of protein32 (Table 1).
Clinical condition and intake.16
| Clinical condition | Non protein energy (kcal/g/day) | Protein or amino acids (g/kg/day) |
|---|---|---|
| Compensated cirrhosis | 25-35 | 1.0-1.2 |
| Complications Inadequate intake Malnutrition | 35-40 | 1.5 |
| Low-grade encephalopathy | 25-35 | Transient 1.0-1.5; if protein intolerant, vegetable protein or BCAA supplement |
| High-grade encephalopathy | 25-35 | 0.5-1.0, BCAA-enriched amino-acid solution |
The above evidence negates the longstanding belief that protein intake can easily result in deterioration of hepatic encephalopathy. Rather, in a double-blind randomized trial to compare oxandrolone and Hepatic Aid II® (BCAA) with a placebo in patients with alcoholic hepatitis, Morgan demonstrated that a high protein intake improved mental status and that a decrease in protein intake was associated with deterioration in mental status.34 This study was the basis for an elegant, well-designed study conducted by Córdoba19 that showed that a very low-protein diet increased protein breakdown and did not have any major benefit in hepatic encephalopathy patients. These results support the hypothesis that long-term protein restriction may worsen the patient’s nutritional status.35
The use of BCAAs, mainly as intravenous solutions, for the treatment of hepatic encephalopathy was established almost 25 years ago. Although a meta-analysis by Naylor suggested that there might be a trend in favor of a beneficial effect of BCAAs, the conclusions of this study are debatable because of differences in study designs, small numbers of patients and short observation periods.36 A Cochrane review then showed that patients supplemented with BCAAs (either intravenous or oral) were more likely to recover from hepatic encephalopathy than patients who received a standard solution and lactulose or neomycin;37 however, survival was not affected by BCAAs. Finally, a meta-analysis by Marchesini38 demonstrated that BCAAs may reduce hospital admissions and the duration of hospital stay, and a meta-analysis by Muto39 showed that BCAAs significantly improved a composite end-point and tended to reduce hepatic encephalopathy. Nonetheless, it is noteworthy that noncompliance with or rejection of BCAAs is a problem of palatability.15
Cirrhotic patients exhibit an early onset of gluconeogenesis after short-term fasting.40 This accelerated metabolic reaction to starvation may underlie their increased protein requirements and muscle depletion. A late evening snack (especially one that contains a BCAA mixture) improves the catabolic state of patients with advanced liver cirrhosis.33 Yamauchi showed that this nocturnal supplement decreases the rate of 3-methylhistidine production by muscle and free fatty acid formation (reflective of muscle and adipose tissue degradation, respectively) and increases albumin production.41
Although the uptake of BCAA supplementation may be limited by noncompliance and its costs, there is increasing evidence that these amino acids may assist intolerant patients to achieve the protein requirement and prevent endogenous protein breakdown; thus, it is a valuable tool in the management of advanced liver disease.15
ConclusionSevere malnutrition is an independent prognostic factor for the survival of patients with cirrhosis. A low protein diet increased protein breakdown and did not have any major benefit in hepatic encephalopathy patients. Vegetable proteins are the best tolerated, followed by proteins contained in dairy foods. The protein requirements of compensated cirrhotic patients consist on a diet containing at least 1.2 g of protein per kg body weight per day. Hepatic encephalopathy should not be a reason to limit the protein content of the diet, and the requirements are 1-1.5 g of protein per kg body weight per day. Patients with severe hepatic encephalopathy (grades III and IV) are exceptions; they may probably benefit of transient protein restriction and should receive 0.5-1 g per kg body weight per day in conjunction with BCAA-enriched amino acid solutions. In conclusion, protein restriction in patients with cirrhosis worsens nutritional status and hepatic encephalopathy and therefore should be avoided.