Non-alcoholic steatohepatitis (NASH) can vary from mild hepatic inflammation and steatosis to cirrhosis, and is most frequently associated with obesity, Type 2 diabetes mellitus, hypertension, and the female gender. The prevalence of fatty liver and NASH in the general population is 20% and 3%, respectively. In Western countries, 15–20% of the population is obese and 74–90% of them exhibit fatty changes in liver biopsies. We assessed the prevalence of NASH in morbidly obese patients and evaluated serum TGF-β1 concentrations in different stages of liver fibrosis. Thirty-five obese patients were evaluated, nine male and 26 female. Their mean body mass index (BMI) was 43.62 ± 7.92 kg/m2. Liver biopsies were evaluated by light microscopy; graded and staged according to Brunt’s system. Serum obtained from patients was used to detect TGF-β1 concentrations by an ELISA method. Serum alanine transaminase (ALT) levels were elevated in four of the patients and the mean level was 49.98 ± 94.7 (8–65 IU/L). NASH was diagnosed in 32 (91%) of the biopsies, and the most common pattern seen was mixed, predominantly macrovesicular steatosis. Some degree of fibrosis was seen in 34 (97%) of the biopsies and 22 (63%) were at stage 2 (range 1–3). Serum concentrations of TGF-β1 had no relationship with the stages of fibrosis. In conclusion, NASH and fibrosis are common in our obese patients, as observed in other studies. TGF-β1 may play a key role in liver fibrogenesis.
Abbreviations:
Key words
TGF-ß1, transforming growth factor ß1
LFT, liver function tests
IntroducciónObesity is a worldwide epidemic that is concerning public health organizations. In Latin America, 20–25% of the population is obese1 and in the United States of America 40% of the Mexican-American population is obese.2 Obesity alone may not be a problem; however, the associated morbidity has a negative effect on health care. Hypertension, diabetes mellitus, coronary diseases, and cerebrovascular accidents are just some examples of the diseases associated with obesity.
Non-alcoholic steatohepatitis (NASH) is a disease of the liver characterized by the presence of fat, inflammation, necrosis, Mallory hyaline bodies and fibrosis seen in liver biopsies.3 NASH has been associated with obesity, diabetes, jejuno-ileal bypass, rapid weight loss, poor nutrition, and with patients who have been put on parenteral nutrition.3-6 The metabolic syndrome X is characterized by insulin resistance, dyslipidemias, hypertension, and maletype distribution of fat.7 There is a high association between metabolic syndrome X and NASH. In Spain, Garcia-MonzÓn et al.8 biopsied 46 obese patients and observed that 69 % had NASH and 41 % had some stage of fibrosis. Ratziu et al.9 studied 93 obese non-alcoholic subjects with similar characteristics and found that 30% had septal fi-brosis and 11% had cirrhosis.
During hepatic fibrogenesis, stellate cells in the liver produce Transforming Growth Factor-β (TGF-β), which controls the extracellular matrix deposition of wound healing proteins such as collagen, fibronectin, proteoglycans, and hyaluronic acid.10,11 This cytokine is also produced by mesangial cells in the kidney, lung cells, and bone. Its main function is to induce wound healing, cell proliferation, and apoptosis. It is also involved in the chemotaxis of neutrophils, monocytes, T lymphocytes, and fibroblasts.10 Tumor necrosis factor (TNF-α), interleukin-6 (IL-6) and TGF-β play important roles in hepatic wound healing and must work all together.13 TNF-α and IL-6 are both pro-inflammatory cytokines, except that the latter is also responsible for hepatocyte development.14 TGF-β was first isolated 10 years ago from platelets.15 There are three isoforms: β1, β2, and β3.16,17 TGF-β 1 is the most abundant form secreted during fibrogenesis.16 It has three cell membrane receptors.18,19 Binding to receptor βRI induces synthesis and deposition of extracellular matrix; βRII activation leads to cellular proliferation and βRIII presents TGF-β to the other two receptors.12 It is possible to detect TGF-β1 in serum by enzyme-linked immunosorbent assay (ELISA) with the help of specific microplates pre-coated with TGF-βRII.20
The aim of this study was to assess the prevalence of hepatic injury in morbidly obese patients and to evaluate serum TGF-β1 concentrations in different stages of liver fibrosis in an attempt to validate its use as an indicator of liver disease progression.
Patients and methodsThe study took place between September 2001 and February 2002 at the University Hospital “Dr. JosÉ E. Gonzalez” of the University of Nuevo LeÓn in Monterrey, Nuevo LeÓn, Mexico. Candidates were chosen to participate in the study based on the following inclusion criteria: body mass index (BMI) >35%, alcohol consumption <30 g/day in men and < 20 g/day in women, age > 18 y, and a negative history of viral, alcoholic, autoimmune or drug-related hepatitis and tumors. Subjects who had been previously treated with amiodarone, corticosteroids, tamoxifen, methotrexate, or high-dose estrogens, or who had received a jejuno-ileal bypass or small bowel resection, or who had received total parenteral nutrition (TPN) were excluded from the study.
Thirty-five subjects who underwent gastric bypass surgery for weight reduction were included. Liver function tests (LFT), basic metabolic panel (BMP), a complete blood count (CBC), lipid profile, and measurements of blood levels of anti-hepatitis B virus (HBV) and C virus (HCV) were ordered for each patient. An abdominal ultrasound was used to rule out any abscesses, tumors, or cysts in the liver before surgery.
This study conformed of Helsinki, and was approved by Hospital´s Research and Ethics Committe. Written informed consent was obtained from all patients.
Liver BiopsyImmediately after the abdominal cavity was opened, a 3 × 3 × 1-cm liver biopsy was taken by excision. Each biopsy was fixed in formaldehyde, embedded in paraffin wax, and stained with hematoxylin and eosin (H & E) and Masson’s Trichrome for the assessment of liver fibrosis. Brunt’s system of the histopathological lesions of NASH was used for the grading and staging of NASH and liver fibrosis.21 The specimens were analyzed by one pathologist with experience in liver pathology.
Serum TGF-β1 MeasurementBefore surgery, a 6-mL sample of venous blood was collected from each patient using an SST tube with EDTA as anticoagulant, and incubated in a 2–8 °C refrigerator overnight to ensure complete release of TGF-β1. Each blood sample was centrifuged at 100 g for 30 min at 4 °C. The obtained serum samples were then stored at –70 °C before the assay. A Quantikine TGF-β1 immunoassay kit (R&D Systems, Inc. Minneapolis, MN) was used. For the activation of latent TGF-β1 to immunoreactive TGF-β1, 0.1 mL of a solution containing 2.5 N acetic acid and 10 M urea was added to 0.1 mL of serum and incubated for 10 minutes at room temperature. The acidified samples were neutralized by adding 0.1 mL of 2.7 NaOH and 1 M HEPES. The activated serum samples were then diluted tenfold. Each sample and standard were pipetted into a previously coated microplate well containing recombinant human TGF-β soluble receptor type II and assayed in duplicate. Each well was washed three times with a buffer solution to wash away any unbound substances, and polyclonal antibodies specific for TGF-β1 conjugated to horseradish peroxide were added to each well. Following a last wash, a substrate solution containing stabilized hydrogen peroxide and tetramethylbenzidine was added to the wells. A bluish color developed in proportion to the amount of bound TGF-β. Sulfuric acid (2 N) was added to stop the reaction and optical density was read using a spectrophotometer at 450 nm corrected to 540 nm. The concentration of the samples was determined based on a standard curve prepared with samples of known concentrations.
Statistical AnalysisData were collected and analyzed using Microsoft Excel 2000 and Primer of Biostatistics software. Averages, medians, modes, and standard deviations of the variables were calculated. Analysis of variance (ANOVA) was usedto test for any significance of differences, and a Bonferroni test to calculate the comparisons between groups.
ResultsDemographyA total of 35 biopsies and serum samples were obtained from a group of morbidly obese patients with a mean BMI of 43.62 ± 7.92 kg/m2. Nine (26%) were men, 26 (74%) were women and their mean age was 33 ± 10 y. Their mean weights and heights were 120.3 ± 28.9 kg and 1.66 ± 0.09 m respectively. In this study, 24% of the patients were known to have hypertension, 22% had fasting glucose levels >110 mg/dL and two were taking oral hypoglycemic drugs at the time of the study.
Laboratory AnalysisLiver function tests (LFTs) were also analyzed. The mean serum level of alanine transaminase (ALT) was 49.98 ± 94.7 IU/L (range 8–65 IU/L) and four of the patients had elevated levels. The mean serum aspartate transaminase (AST) level was 35.18 ± 60.23 IU/L (range 7–40), and three had elevated levels of the enzyme. Mean serum lactate dehydrogenase and alkaline phosphatase levels were 239.3 ± 128.29 IU/L (range 100–225) and 99.4 ± 40.7 IU/L(range 30–140), respectively. Only one patient had significant elevation of all the liver enzymes measured.
Histopathological AnalysisNASH
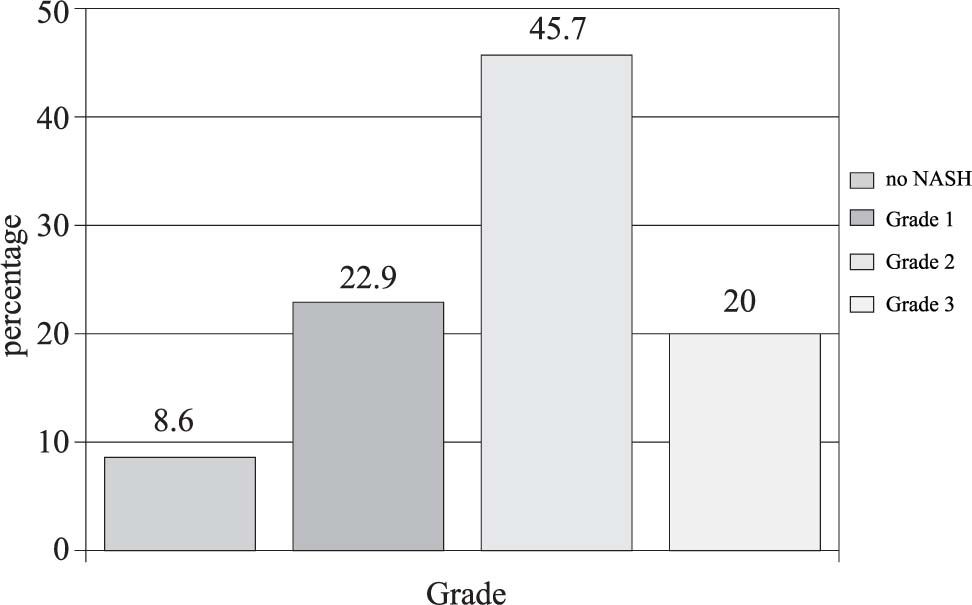
Thirty-five biopsies were analyzed and classified according to Brunt’s grading and staging system. The most common pattern of hepatic steatosis found in the biopsies was a predominantly macrovesicular steatosis mixed with microvesicular steatosis (43%). A mixed predominantly microvesicular steatosis pattern was present in 20% of the specimens. Chronic lobular inflammation was present in 16 (46%), and 31 (89%) presented acute lobular inflammation. Mild portal inflammation was present in 18 (51%), moderate portal inflammation in 13 (37%) and one (3%) had no portal inflammation. Grade 1 NASH was present in eight (23%), grade 2 in 16 (46%) grade 2 and grade 3 in seven (20%). Three biopsies (9%) could not be classified into a group (Figure 1).
Fibrosis
Thirty-four patients (97%) had some degree of fibrosis. Stage 1 fibrosis was present in six patients (17%), stage 2 in 22 (63%) and stage 3 in five (14%). In this category, only one patient (3%) did not have fibrosis (Figure 2). ALT levels and Stages of Fibrosis The ALT levels were compared between the three stages of fibrosis using a Bonferroni t test (Figure 3). Significant statistical difference was found when comparing ALT levelsbetween Stage 2 and Stage 3 of fibrosis (p = 0.03, t =2.763). Not all subjects had elevation of ALT and AST levels(12% and 9% were elevated, respectively).
Serum TGF-β1 concentrationSerum concentrations of TGF-β1 were grouped according to the stage of fibrosis, calculating the mean concentration (ng/mL) of each group, and a comparison was made between them to search for any difference betweenthe groups (Figure 4). The concentration for stage 1 fibrosis was 36.77 ± 6.16 ng/mL.For stage 2 fibrosis the concentration was 41.57 ± 10.45 ng/mL, and it was 37.45 ± 7.83 ng/mL for stage 3. No statistically significant differences were seen between groups.
DiscussionTGF-β1 is just one of the many serum markers that could potentially be used for a non-invasive diagnosis of hepatic fibrosis. Matrix deposition proteins, matrix production and degradation enzymes, and other fibrogenic cytokines could be used to assess this process in the liver. Among the members of the matrix molecule family, laminin, hyaluronic acid, and collagen type I and IV have been the most studied and have the most potential clinical utility.22,23 Levels of other fibrogenic cytokines such as platelet-derived growth factor (PDGF) and connective tissue growth factor (CTGF) might also be clinically useful.
The results in the obese population we studied were similar to most of the published literature. A high prevalence of NASH was found in this group of patients. In our study, 32 (90.4%) had some grade of NASH and some degree of fibrosis was present in nearly all of them (97%). NASH is not always intentionally looked for when LFTs are within normal values in obese patients. As a high proportion of our population studied was diagnosed with NASH and yet the ALT levels were normal in 83%, routine LFT and ALT values evidently lack sensitivity for this condition.
We do not have any doubts as to the importance TGF-β has in the mechanism of wound healing and hepatic fibrosis. TGF-β1 serum concentrations were compared between fibrosis stage and grade groups, and no significant differences were found among them (Figure 4). Our results show that even though TGF-β1 is not distributed evenly among the three stages of fibrosis, it is possible to detect it in serum analysis. Therefore, our study suggests that TGF-β1 is not the best serum marker for this particular subgroup of patients. A well-selected control group should be included to further complement our results.
The diagnosis of NASH currently represents a clinical challenge and we must take into account several parameters such as anthropometric measurements, insulin resistance, blood pressure, and dyslipidemias to find it when studying obese patients and assessing the presence of this condition.24
Genetic factors may contribute to insulin resistance, and TNF-α promoter polymorphisms may influence it, leading to non-alcoholic fatty livers and steatohepatitis.25
We conclude that liver biopsy remains the “gold-standard” for diagnosing, grading, and staging liver diseases and a more sensitive serological marker is necessary to help us identify these patients before they develop terminal liver disease.