Intravenous albumin infusion prevents complications after large-volume paracentesis (LVP), particularly paracentesis-induced circulatory dysfunction (PCD), and improves patient survival. However, albumin is expensive. We compared a low-molecular weight dextran (Dextran-40) with albumin in treating LVP in cirrhotic patients with tense ascites. Sixty-nine cirrhotic patients were included and 96 LVPs were performed. Any repeat punctures on the same patient were at least three months apart. Patients were randomized to receive either i.v. Dextran-40 infusion (Group I, n = 48) or i.v. albumin infusion after LVP (Group II, n = 48). Clinical, biochemical, and hormonal evaluations were done before and after LVP. Patients were followed up for the detection of any recurrence of ascites or complications.The two groups were similar in age, sex, and etiology of cirrhosis, and in the volumes of ascites recovered. Significant decreases in mean arterial pressure were observed in both groups 24 and 48 h after LVP. Urine volumes increased significantly at 24 h in both groups (p < 0.05), but remained high only in Group I. Plasma renin activity and aldosterone concentrations increased in both groups 48 h after LVP, but they were more marked in Group I. Complications developed in 17 % of patients treated with Dextran-40 and in 23 % treated with albumin (p > 0.05). Ascites recurrence rates and survival were similar in the two groups. In conclusion, Dextran-40 was thus not as efficacious as albumin for preventing PCD.
Therapeutic paracentesis involving either repeated large-volume (> 5 L per puncture) or total paracentesis (complete mobilization of ascites with only one puncture), associated with intravenous albumin infusion is a safe and efficient treatment for tense, recurrent, or refractory cirrhotic ascites.1–3 This intravenous infusion of albumin seems to be essential for avoiding complications (renal impairment, hyponatremia, hepatic encephalopathy, and paracentesis-induced circulatory dysfunction (PCD).4–7 PCD is a manifestation of hypovolemia that induces a marked increase of the degree of activity of the renin-angiotensin and sympathetic nervous systems.8 It generally occurs between the second and sixth days after paracentesis and may be prolonged over weeks and months. It is associated with an early recurrence of ascites and a reduction in long-term survival.8 Other plasma expanders may not be as efficient as albumin for avoiding PCD.8,19,20,21 In a recent study, PCD occurred with a significantly greater frequency in patients treated with Dextran-70 (34.4 %) and Polygeline (37.8 %) than in those treated with albumin (18.5 %).8 However, albumin is expensive, even for clinicians in developed countries. This prompted us to explore the potential uses of less expensive and more available plasma expanders with the aim of preventing post-paracentesis complications.
In this randomized controlled trial, we compared the effects of infusing a low-molecular weight dextran (Dextran-40) with those of albumin in treating LVP in cirrhotic patients with tense ascites.
Experimental proceduresPatientsCirrhotic patients with tense ascites were included in this trial. They were admitted to the Hepatogastroenterology Service of Saint-Eloi Hospital in Montpellier and the Gastroenterology Service of the Hospital of Tulle, France. Tense ascites was determined as that causing respiratory dysfunction.
The following criteria, previously established by the Barcelona Group,5 were required for inclusion: a) absence of clinical, biochemical, or echographic data suggesting hepatocellular carcinoma; b) absence of hepatic encephalopathy, gastrointestinal hemorrhage, and either local or systemic infection; c) serum bilirubin levels < 10 mg/dL; d) prothrombin time > 40 %; e) platelet count > 50,000 mm;3 f) serum creatinine < 3 mg/dL; and g) serum sodium > 125 mEq/L. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Hospital’s Research and Ethics Committee. Written informed consent was obtained from all patients participating in this trial.
Patients were admitted to the hospital at least 5 d before treatment. Diuretics were discontinued 3 d before treatment. A standard diet containing less than 50 mEq of sodium per day was prescribed in all patients, and fluid restriction (800 mL/d) was carried out only in patients whose serum sodium concentrations were lower than 130 mEq/L. Other drugs were excluded except for lactulose, antacids, H2 receptor blockers, and antibiotics when clinically indicated.
Patients were randomly assigned into two therapeutic groups using a random number table. Those in Group I were treated with LVP plus an i.v. Dextran-40 infusion, while those in Group II were treated with LVP plus an i.v. albumin infusion. Eight grams of either albumin or dextran were given for each liter of ascites removed.
ParacentesisUsing aseptic precautions, paracentesis was performed either in the left or in the right lower abdominal quadrant using an 18-gauge needle. Ascitic fluid was drawn by gravity into a bottle resting on the floor. The time required for completing the procedure varied from 45 to 90 min. Samples of ascitic fluid were taken for cell count, culture, and protein and electrolyte determinations. As soon as the procedure was started, either a solution of 20 % human albumin containing a sodium concentration of 80 mEq/L (Centre National de Transfusion Sanguine, Montpellier, France) or a sodium-free low-molecular weight Dextran-40 (M Wt 40000)-sorbitol solution (Rheomacrodex, Kabi Pharmacia) were infused for 1 or 2 h. The Dextran solution contained 10 g of Dextran-40 and 5 g of sorbitol per 100 mL.
EvaluationsClinical, biochemical, hemodynamic, and hormonal evaluations were performed before and after paracentesis: body weight, mean arterial pressure (MAP), heart rate (HR) and urinary volume were assessed before paracentesis, and 24 and 48 h later. Blood samples were drawn to measure serum electrolytes, blood urea nitrogen (BUN), serum creatinine, blood cell count, and to perform standard liver function tests. Renal impairment was defined as an increase > 50 % in serum creatinine or BUN after treatment, and hyponatremia was defined as a decrease of > 5 mEq/L after treatment. PCD was defined as an increase of > 50 % in plasma renin activity (PRA) from the pre-treatment value to a level of > 4 ng/mL per hour.8
On the day that paracentesis was carried out, the patients, having lain supine overnight, had blood samples taken in order to determine PRA and PAC 2 h before paracentesis. Similarly, levels were measured 2 d after paracentesis. Blood samples were collected in EDTA-treated tubes, centrifuged at 2500 rpm for 10 min at –3ºC, and stored at –20ºC until assayed. PRA was measured by the generation of angiotensin I with an incubation period of 1.5 h, using a Sorin-Ria kit (RENCTK; Sorin Biomedica, France). PAC was determined by a competition reaction between 125I-labeled aldosterone and the aldosterone contained in specimens for a fixed and limited number of antibody binding sites bound to the solid phase, using an Aldo-CT kit (CIS Bio International, France).9,10 Normal values for our laboratory are PRA, 0.2–2.8 ng/mL per hour, and plasma aldosterone concentrations (PAC), 6–12 ng/mL.
Plasma volume was measured in 25 patients from Group I and 16 from Group II, using the 51Cr-labeled human red blood cell dilution technique, both before and 48 h after paracentesis.11
Statistical AnalysisThe Kruskall-Wallis test was used to compare continuous variables. Chi-squared or Fisher’s exact tests were used to compare qualitative variables. Two-way analysis of variance with one factor fixed, and one repeated-measures analysis were used to test for time-dependence or treatment-dependence (Dextran-40 or albumin) in any changes in continuous variables. The cumulative probability of ascites recurrence and the cumulative probability of survival curves were compared using a log rank test. Statistical significance was assumed at p < 0.05. All these calculations were done using the BMDP program.12
ResultsPatients Sixty-nine patients were included in this trial, to whom 96 LVP were performed: 48 with Dextran-40 infusion (Group I) and 48 with albumin infusion (Group II). Histological confirmation of cirrhosis was performed in all cases. In 57 patients, the etiology of cirrhosis was due to alcohol, in five cases it was due to a viral infection. Three patients had primary biliary cirrhosis (PBC). One patient had autoimmune cirrhosis, and in three patients, the etiology was not determined. Fifty-four patients were males and 15 were females. Their mean age was 58 ± 10 years. In none of the patients was confirmation of the refractoriness of the ascites by previous intensive treatment with diuretics performed. Thirty-nine patients received at least one LVP associated with Dextran-40 infusion and 35 received at least one LVP plus albumin infusion. Distribution of the 96 LVPs either with Dextran-40 (Group I) or with albumin (Group II) in the 69 patients was as follows: one LVP, 52 patients (29 in Group I and 23 in Group II); two LVPs, 10 patients (four in Group I, four in Group II, and two in both groups consecutively). Multiple punctures to the same patient were at least three months apart. In such cases, the patients were re-randomized to be re-assigned to one of the two treatments. Accordingly, the punctures were considered as independent events and were analyzed separately. Therefore, clinical, biochemical, hemodynamic, and humoral parameters were assessed for each LVP. There were no significant statistical differences between the two groups in any clinical or biochemical parameters before treatment (Table I).
Clinical and biochemical data of patients at the time of inclusion to the study. values are given for 96 punctures (48 with Dextran-40 and 48 with albumin). Values are expressed as means ± SD.
| Dextran-40 n = 48 | Albumin n = 48 | p | |
|---|---|---|---|
| Age (years) | 59.4± 11.2 | 58.9±15.9 | NS |
| (range) | (39 - 88) | (22 - 88) | |
| Gender (M/F) | 35 / 13 | 34/14 | NS |
| Alcoholic cirrhosis (n) | 38 | 39 | NS |
| Renal impairment (n) | 15(31 %) | 12(25 %) | NS |
| Body weight (kg) | 69.6 ± 14.7 | 70.3±13.3 | NS |
| Mean arterial pressure (mm Hg) | 87 ± 12 | 89±11 | NS |
| Serum bilirubin (mg/dL) | 2.8 ± 2.9 | 3.32±3.95 | NS |
| Prothrombin (%) | 56.6± 13.9 | 57.2± 14.7 | NS |
| Serum albumin (g/L) | 2.9 ± 0.5 | 3.0±0.5 | NS |
| BUN (mg/dL) | 32 ±22.9 | 34.1±24.1 | NS |
| Serum creatinine (mg/dL) | 0.9 ±0.3 | 0.8±0.3 | NS |
| Serum sodium (mEq/L) | 135.9 ±5.4 | 135.7±4.6 | NS |
| Serum potassium (mEq/L) | 4.1 ± 0.7 | 3.9±0.5 | NS |
| Urine volume (mL/d) | 461.4 ± 272.5 | 613.5±592.8 | NS |
| PRA (ng.ml-1h-1) (a) | 11 ± 9.5 | 11.8±6.4 | NS |
| PAC (pg/dL) (b) | 42.8 ±25.5 | 46.7±41.6 | NS |
| Extracted volume, L (mean ± SD) | 5.7 ± 1.4 | 5.2±0.4 | NS |
| (range) | (5 - 10) | (5-7) | NS |
| Plasma volume (mL) (c) | 3189± 654.4 | 3310±770 | NS |
| Child-Pugh score | 10.3 ± 1.3 | 10±1.5 | NS |
Most patients had advanced liver disease, as indicated by a high Child-Pugh’s score (10.3 ± 1.3). Serum bilirubin was increased (> 1 mg/dL) and prothrombin activity was reduced (< 80 %) in 78 % of the patients. Serum albumin concentration was reduced (< 35 g/L) in 92 % of patients. At the time of inclusion into the study, 15 patients from Group I (31 %) and 12 from Group II (25 %) had renal impairment, and five patients from Group I (10 %) and six from Group II (12 %) had hyponatremia.
The two groups did not differ with respect to the volume of ascites removed (Group I, 5.7 ± 1.4 L, range 5–10 L; Group II, 5.2 ± 0.4 L, range 5–7 L), the mean duration of paracentesis (70 ± 15 min in Group I vs. 63 ± 18 min in Group II), or the duration of hospitalization (9.5 ± 2.5 d in Group I vs. 9 ± 3 d in Group II). No local complications related to paracentesis were observed in any patient.
The effects of treatment on body weight, MAP, standard liver and renal function tests, serum electrolytes, and urinary and plasma volumes are shown in Table II for Group I. Significant changes were not observed at 24 or 48 h in serum biochemical parameters, particularly BUN, creatinine, sodium or potassium. Plasma volume values remained similar. Body weight was significantly better than that observed at the baseline period.
Clinical and biochemical data of patients in the group treated with Dextran-40 before and after paracentesis (x ± SD).
| Baseline | 24 h | 48 h | |
|---|---|---|---|
| Body weight (kg) | 69 ± 15 | 64 ± 15 (a) | 64 ± 14 (a) |
| MAP (mm Hg) | 87 ± 12 | 82 ± 12 (a) | 81 ± 12 (a) |
| BUN (mg/dL) | 35 ± 23 | 34 ± 20.5 | 34.7 ± 21.1 |
| Serum creatinine (mg/dL) | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.9 ± 0.3 |
| Serum sodium (mEq/L) | 136 ± 5 | 136 ± 5 | 135 ± 5 |
| Serum potassium (mEq/L) | 4.1 ± 0.7 | 4.2 ± 0.7 | 4.1 ± 0.7 |
| Urine volume (mL/d) | 461 ± 273 | 1003 ± 545 (b) | 623 ± 400 (c) |
| Plasma volume (mL) | |||
| (mean ± SD) | 3189 ± 655 | 3380 ± 957 |
The effect of treatment on body weight, MAP, standard liver and renal function test results, serum electrolytes, urinary volume, and plasma volume is shown in Table III for Group II. Significant changes were not observed at 24 or 48 h in biochemical parameters, except for BUN, which increased significantly at 48 h (p < 0.05 vs. baseline and 24-h values). Creatinine, sodium, and potassium levels in serum remained unchanged. Body weight and MAP decreased significantly 24 and 48 h after treatment. Urine volumes increased significantly at 24 h, but decreased at 48 h after treatment to a volume similar to that observed at baseline.
Clinical and biochemical data of patients in the group treated with albumin: before, 24 h and 48 h after paracentesis (x ± SD) (n = 48).
| Baseline | 24 h | 48 h | |
|---|---|---|---|
| Body weight (kg) | 70 ± 13 | 65.4 ± 13 (a) | 62 ± 13 (a) |
| MAP (mm Hg) | 89 ± 11 | 84 ± 11 (a) | 84 ± 10 (a) |
| BUN (mg/dL) | 34 ± 24 | 35.2 ± 25.2 | 39.4 ± 36 (b) |
| Serum creatinine (mg/dL) | 0.8 ± 0.3 | 0.8 ± 0.4 | 0.8 ± 0.4 |
| Serum sodium (mEq/L) | 136 ± 5 | 136 ± 5 | 136 ± 5 |
| Serum potassium (mEq/L) | 3.9 ± 0.5 | 4 ± 0.5 | 4 ± 0.5 |
| Urine volume (mL/d) | 612 ± 593 | 904 ± 502 (c) | 683 ± 415 (d) |
| Plasma volume (mL) | |||
| (mean ± SD) | 33100 ± 770 | 3483 ± 753 |
PRA and PAC were assessed in 19 and 21 patients from Group I, and 16 and 15 patients from Group II, respectively. PRA was abnormally increased (above the upper normal value) in 14 patients from Group I (74 %) and 14 (88 %) from Group II; PAC was abnormally increased in 20 patients from Group I (95 %) and 14 (93 %) from Group II before treatment.
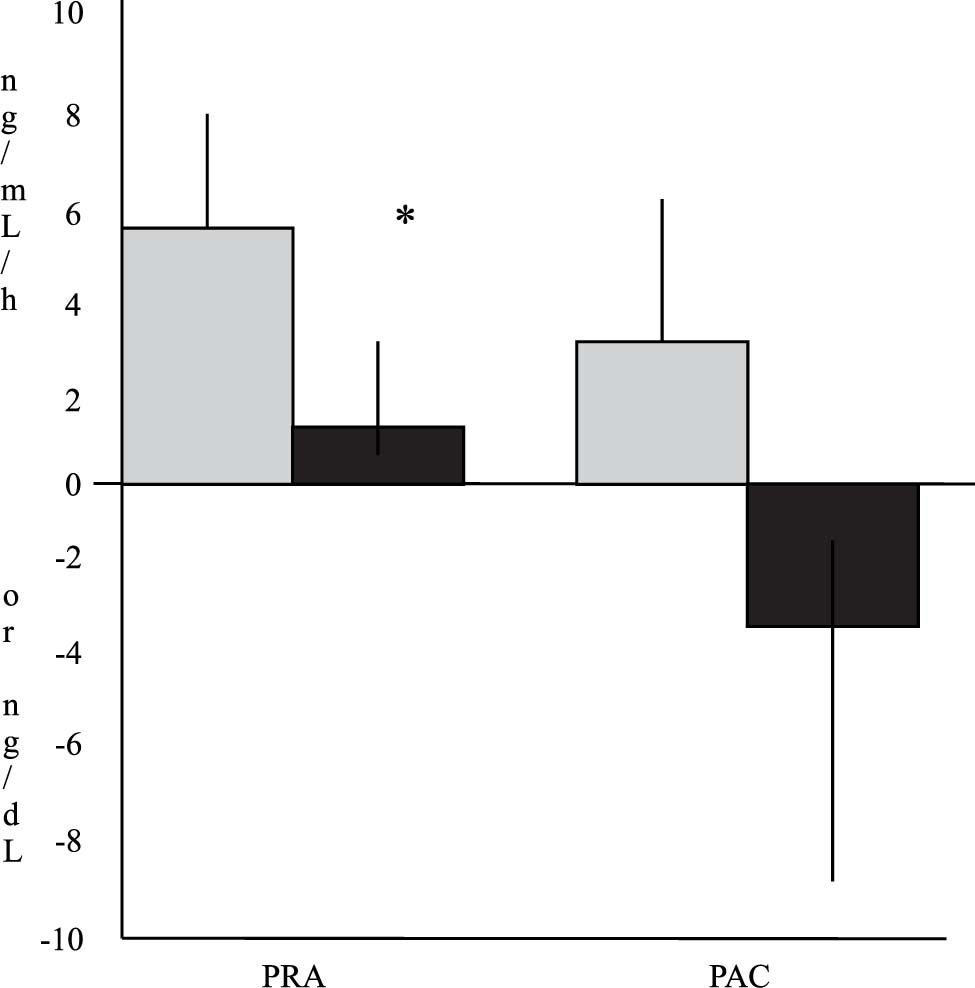
The mean changes in these hormones from the baseline values are depicted in Figure 1. PRA increased in both groups. This change was more marked in Group I (6 ± 8.3 ng/mL per hour in Group I vs. 1.5 ± 7.7 ng/mL per hour in Group II). Differences between values of both groups reached statistical significance (p = 0.04). Similarly, PAC showed an increase from the baseline values in Group I and a decrease bellow the baseline values in Group II after treatment (3.1 ± 20.2 ng/dL in Group I vs. –3.2 ± 22 ng/ dL in Group II). These differences did not reach statistical significance (p = 0.37).
Changes in plasma renin activity (PRA) and plasma aldosterone concentrations (PAC) 2 d after large-volume paracentesis in relation to baseline values. The gray bars indicate patients from Group I (with Dextran-40, n = 19 for PRA and n = 21 for PAC), and the black bars indicate patients from Group II (with albumin, n = 16 for PRA and n = 15 for PAC). The values are expressed as means ± SEMs (* p < 0.05 between groups).
Individual values of the PRA of the patients are shown in Figure 2. PCD was observed in 42 % of patients treated with Dextran-40 and in only 20 % of patients receiving albumin at 48 h after paracentesis (p < 0.05 by Chi-squared test).
Plasma volume remained similar after treatment in both groups: 3189 ± 770 mL in Group I vs. 3483.1 ± 753 mL in Group II (p > 0.05).
Complications developed in eight patients (17 %) treated with Dextran-40, and in 11 (23 %) treated with albumin. There were no significant differences in the type of complications occurring in both groups (Table IV). In two patients from Group I (4 %) and in seven cases from Group II (14 %), renal impairment developed after treatment. Hyponatremia developed in five patients treated with Dextran-40 and in three treated with albumin. This was asymptomatic in all eight patients and improved spontaneously during the hospitalization period. No patients died during hospitalization.
Complications after treatment in both groups of patients.
| Variables | Dextran-40 | Albumin | p |
|---|---|---|---|
| n = 48 | n = 48 | ||
| Patients with complications | 8 (16 %) | 11 (22 %) | NS |
| Hyponatremia | 5 (10 %) | 3 (6 %) | NS |
| Hyperkalemia | 4 (8 %) | 0 | NS |
| Renal impairment | 2 (4 %) | 7 (14 %) | NS |
| Local hematoma | 1 (2 %) | 0 | NS |
| Fever | 0 | 1 (2 %) | NS |
All patients were discharged from the hospital with prescriptions of a low sodium content diet and for diuretics to reduce the risk of ascites recurrence.
The mean follow-up period in all patients was 14 ± 11.8 months (range 0.5–44 months). In Group I the mean follow-up period was 13.7 ± 11.7 months (range 0.5–44 months), and in Group II the mean follow-up period was 14.4 ± 12 months (range 0.5–40 months). Sixty-four patients required readmission during follow-up for ascites recurrence: 34 (71 %) from Group I and 30 (62 %) from Group II. Readmission occurred at a mean of 54 d (95 % confidence intervals, 42–72 d) after discharge in patients treated with albumin. There were no significant statistical differences between both groups for either the frequency or the delay of ascites recurrence.
Twenty-nine patients died during the follow-up period: 18 from Group I, and 11 from Group II (p > 0.05). The causes of death were: gastrointestinal hemorrhage in seven patients from Group I and four from Group II; sepsis in four from Group I and two from Group II; hepatorenal syndrome in three from Group I and one from Group II; hepatocellular carcinoma in two from Group I and one from Group II; and the cause was undetermined in two patients from Group I and three from Group II. There were no statistically significant differences between the groups, and the survival curve of patients was not different between both groups (Figure 4).
DiscussionThe importance of the use of plasma expanders associated with LVP has been debated.13–15 Some studies have shown hemodynamic and hormonal changes (significant reductions in cardiac output, central venous pressure, and pulmonary capillary wedge pressure, and increments of plasma renin activity and plasma aldosterone), suggesting effective hypovolemia after LVP without the use of plasma expanders.3,16,17 Albumin infusion (8 g for every liter of ascites removed) immediately after paracentesis prevented these changes.3
These hemodynamic and hormonal abnormalities may be related to a high incidence of complications, particularly PCD, which is defined as an increase of greater than 50 % in PRA from baseline values.5–8 PCD may be associated with an early recurrence of ascites and a long-term survival reduction.8 In one study, this abnormality was observed in 75 % of patients not receiving plasma expansion, and in 15 % of patients treated with albumin.7 Hyponatremia and renal impairment were more frequent in patients without albumin infusion. Survival was not different between these groups of patients.7 The authors concluded that albumin is necessary to reduce complications after paracentesis.
However, the use of albumin is limited by its high cost. Therefore, the use of less expensive plasma expanders was recently investigated.8,19,20,21
One study compared a high-molecular weight dextran (Dextran-70) with albumin in a randomized investigation of 88 patients. The incidences of hyponatremia, renal impairment, and survival were similar in both groups. Nevertheless, 51 % of the patients treated with Dextran-70 showed hypovolemia (as estimated by an increase of PRA) compared with only 15 % of patients receiving albumin. 21
In another randomized controlled trial, albumin was compared with other synthetic plasma expanders (Dextran-70 and Polygeline). Albumin was significantly superior to the other solutions in preventing PCD. These significant differences were observed only when the volume of ascites removed was > 5 L. Below these levels, all solutions were similarly effective. Nevertheless, after 10 months of follow-up, no significant differences were found among the groups in the numbers of patients requiring readmission and in the incidence of death.8
Dextrans are polysaccharides consisting of glucose molecules connected by glucosidal linkages. Dextran-40 and Dextran-70 are the most frequently used solutions of this type. Dextran-40 has a molecular weight ranging between 10,000 and 80,000, which is lower than that of Dextran-70 (15,000–160,000). The smaller molecules are more rapidly eliminated by the kidney, so Dextran-70 has a higher elimination half-life. In normal subjects, this ranges from 23 to 36 h, whereas it ranges from 10 to 14 h for Dextran-40.22,23
Dextran-40 was not as efficacious as albumin in preventing PCD in cirrhotic patients with tense ascites. Ascites was observed in 42 % of patients with Dextran-40, whereas it was observed in only 20 % of those treated with albumin (p < 0.05). However, we did not observe more complications after Dextran-40 infusion than after albumin infusion, with the probability of readmission to the hospital for ascites recurrence and the probability of survival being similar in both groups. Similar results have been observed in previous studies comparing Dextran-70, Polygeline or Hemaccel with albumin.8,19,20,21 Nevertheless, the incidence of PCD in our study was higher than that observed with Dextran-70 and similar to that seen with Polygeline. The explanation of this may be due to the very short biological half-life of Dextran-40 (10–14 h), which may be insufficient to protect patients from the PCD that appears from the second to the sixth day after paracentesis. In fact, the shorter the half-life of any solution, the higher the risk of developing PCD (Dextran-40, 10–24 h; Dextran-70, 23–36 h; albumin 21 d: (Figure 4).
There are some important points regarding our study. First, we did not evaluate the incidence of early recurrence of ascites nor survival among patients with PCD compared with those without PCD from both groups. As previously mentioned, these issues have been evaluated in other studies that show that this abnormality is associated with a higher frequency of recurrence of ascites and a reduction in the probability of survival compared with patients without PCD.8,24 Second, the absence of significant differences of complications, and survival, in our study was similar to other studies that evaluated synthetic plasma expanders (Dextran-70, Polygeline and Hemaccel) against albumin.8,19,20,21 Nevertheless as shown in some of these studies, long-term complications and deaths related to renal dysfunction were more frequent in patients treated with these solutions as well as with Dextran-40 (in our study) than in patients treated with albumin. The number of patients in our study was small. Probably, more studies with higher sample sizes are needed to reach significant conclusions regarding complications and survival.
In conclusion, Dextran-40 was not as efficacious as albumin for preventing PCD after LVP. However, the frequency of complications, long-term recurrence of ascites, and survival were similar after both treatments.
Since PCD may have a deleterious effect on survival, we actually recommend the use of albumin when the volume of ascites removed is > 5L. It seems reasonable to use a less expensive plasma expander (Dextran-70, Dextran-40, Polygeline or Hemaccel) when the volume of ascites removed is less than 5 L. Because albumin is expensive, in the future, more studies are needed to clarify the precise role of albumin, particularly studies that target survival as the specific study endpoint.