Objective. To systematically review the effect of Acetyl-L-Carnitine in patients with hepatic encephalopathy.
Material and methods. Design: systematic review and meta-analysis. Data sources: The Cochrane Library, MEDLINE, EMBASE.com, Science Citation Index, Google search and the China Biological Medicine Database to June 2012.
Review methods: randomized placebo controlled trials of Acetyl-L-Carnitine in patients with hepatic encephalopathy assessing whether Acetyl-L-Carnitine an effective therapy or not. No language restrictions were applied. Two reviewers independently extracted data and assessed quality.
Results. 7 methodologically sound randomized controlled trials were identified involving 660 participants with hepatic encephalopathy, totaling 249 with subclinical hepatic encephalopathy, 189 with West Haven grade 1, 162 with West Haven grade 2 and 60 with West Haven grade 3. Acetyl-L-Carnitine was effective to improve serum ammonia level (weighted mean difference 25.90, 95% confidence intervals 20.89 to 30.91, P < 0.05) and the number connection test completion time (weighted mean difference 16.62, 95% confidence intervals 9.88 to 23.36, P < 0.05). The outcome was consistent in subgroup analyses. No publication bias was detected. Adverse events were reported infrequently and were minor.
Conclusions. Acetyl-L-Carnitine is promising as an effective and tolerable treatment for hepatic encephalopathy that associated with improved serum ammonia levels and the number connection test.
Hepatic encephalopathy (HE) represents a broad neuropsychiatric syndrome that arising from a consequence of acute or chronic hepatic failure.1 Its symptoms may or may not be clinically apparent, but always impair function and quality of life. The main pathophysiological mechanism of HE are based upon evidence of the accumulation of toxic substances, including the ammonia, glutamine, manganese, false neurotransmitters, inflammation, short chain fatty acids, oxidative stress, mercaptanes, neurosteroids, or low grade edema.2 However, the most widely accepted is that accumulation of endogenous and gut-derived ammonia crossing the blood-brain barrier, and functional changes in various neurotransmitter systems.3
Thus, current therapies focus on pathogenesis previously accepted, and the roles of drugs were critically estimated through systematic review according to methodology of Evidence-based Medicine (EBM), such as non-absorbable disaccharides,4 rifaximin,5 L-ornithine-L-aspartate (LOLA),6 naloxone7 have been widespread used now. Over the last two decades, studies concerning use Acetyl-L-Carnitine (ALC) have demonstrated beneficial effects.8 L-carnitine is essential for the transfer of long-chain fatty acids from the cytoplasm to the inner mitochondrial membrane, thereby facilitating mitochondrial energy metabolism. ALC represents an ester of the tri-methylated amino acid and is synthesized in the human brain, liver and kidney by the enzyme ALC-transferase. It can cross the blood-brain barrier and reach the cerebral regions, where the acetylic group may be used. In addition, ALC facilitates the uptake of Acetyl-CoA into the mitochondria during fatty acid oxidation, enhances acetylcholine production, stimulates protein and membrane phospholipids synthesis, and provides a substrate reservoir for cellular energy production, thereby preventing excessive neuronal cell death.9 According to their metabolic functions and neurophysiological roles, L-carnitine and its acetylated derivate, ALC are suggested as a therapeutic agent in several neurological disorders, including HE.10 Some reports have indicated efficacy of ALC in HE, however, questions remain as to its systemic versus cerebral effects, its relative effects on astrocytes and neurons, and its clinical use.
Therefore, we undertook a systematic review and meta-analysis based on view of EBM to evaluate available randomized controlled trials (RCTs) on the effectiveness and safety of ALC in the treatment of HE.
Material and MethodsA systematic review of the literature was carried out according to PRISMA guidelines11 for the conduct of meta-analysis of intervention trials. Electronic databases including the Cochrane Controlled Trials Register on the Cochrane Library, MEDLINE, EMBASE.com, Science Citation Index, Google search and the China Biological Medicine Database (CBMdisc) (January 1978-June 2012) using the terms “carnitine”, “L-acyl-carnitine”, “hepatic encephalopathy” and by limiting the searches to RCTs were searched with a cutoff date of 30 June, 2012. Reference lists of pertinent reviews and retrieved articles were also checked for additional studies identification.
Eligibility criteriaInclusion criteria were:
- •
RCTs compared oral ALC to placebo, irrespec tive of medication, blinding, publication status or language published as full-length articles in peer-reviewed journals.
- •
The diagnosis of HE was based on clinical, biochemical and/or liver histological data and the grade was on the widely used West-Haven criteria introduced by Conn.12
Exclusion criteria were:
- •
Non-randomized trials and observational studies.
- •
Repetitive reports (if more than one version of the same study was retrieved, only the most recent one was used.
- •
Results published only in an abstract.
- •
The presence of major psychiatric illness, chronic renal and/or respiratory insufficiency, tumors, inter-current infections, pregnant or lactating women, and patients who did not fulfill protocol requirements.
- •
No additional interventions except ALC were allowed. All articles were reviewed by two independent reviewers using the inclusion and exclusion criteria.
Using a standardized form two reviewers extracted data on participant characteristics (study population, type of HE, intervention characteristics, mean duration of follow-up, and outcome events). Data were extracted according to intention to treat principles. Quality of included reports was scored using the Jadad composite scale,13 which assesses the description of randomization, blinding, and withdrawals in the reports.14 The quality scale ranges from 0 to 5 points with a low quality report of score 2 or less and a high quality report of score at least 3.15 All discrepancies were resolved after going back to the original source papers, by consensus and involvement of a third reviewer when necessary.
Data synthesis and analysisSerum ammonia concentration and the number connection test (NCT) are both components of the widely used porto-systemic encephalopathy (PSE) index according to the West-Haven criteria.16 Considering the improvement might be significantly different for patients divided into different West Haven grades, subgroup analyses were performed by for dif ferent groups according to the West-Haven criteria.
The effect measures estimated were relative risk (RR) for dichotomous data, and weighted mean difference (WMD) for continuous data, using 95% confidence intervals (CIs). Pooled RR or WMD was calculated using the general inverse variance fixed effect model. The heterogeneity between studies was examined by DerSimonian and Laird (DL) Q statistical analysis.17 The I2 statistic was used to estimate the percentage of variability across studies attributable to heterogeneity beyond chance. If results were heterogeneous (P < 0.05), a random effects model was employed using the DL methods. Begg and Mazumdar’s18 proposed adjusted rank correlation test and Egger’s linear regression approach were used to measure publication bias,19 which was shown as a funnel plot.
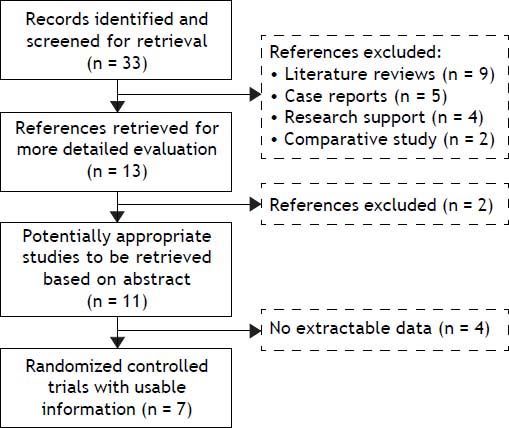
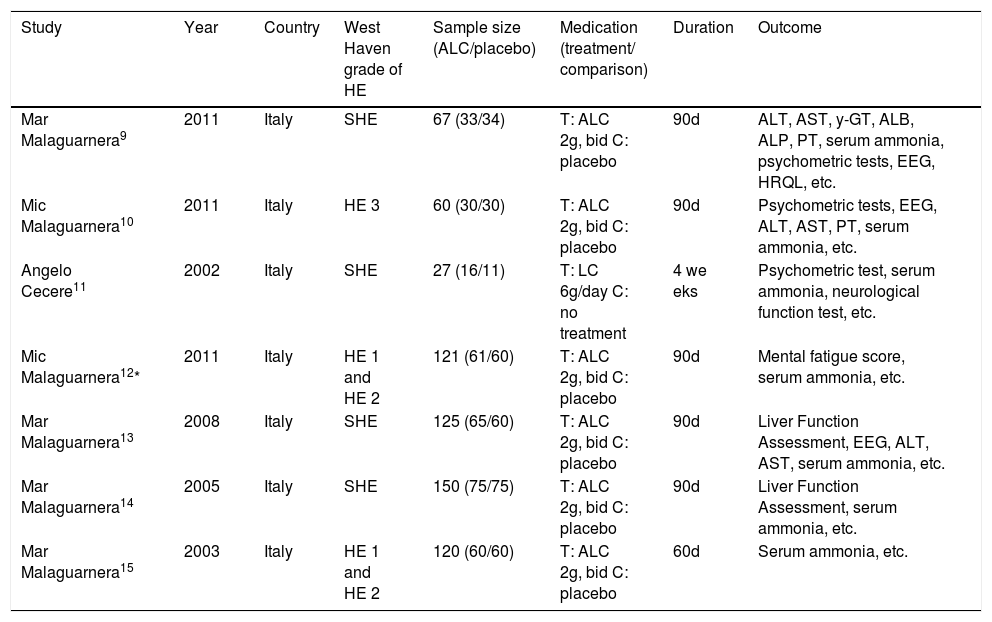
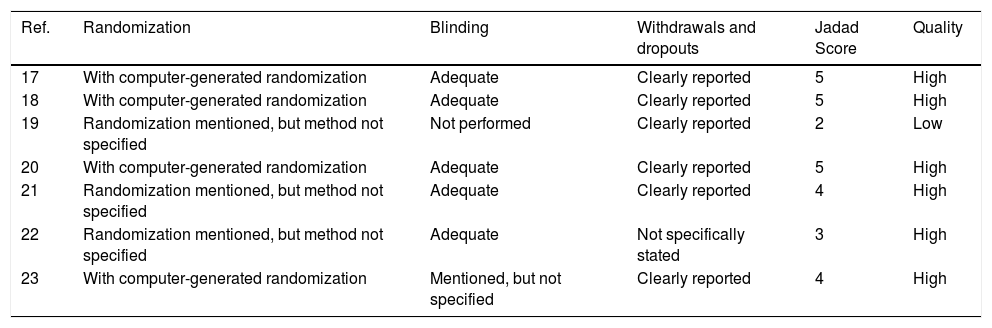
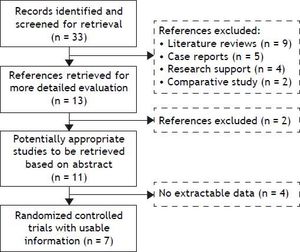
ResultsThe search yielded 33 articles, of which 11 were reviewed in full text (Figure 1). 7 randomized trials19–25 provided information on 660 participants with HE, totaling 249 with subclinical hepatic encephalopathy (SHE), 189 with West Haven grade 1, 162 with West Haven grade 2 and 60 with West Haven grade 3 based on the widely used West-Haven criteria introduced by Conn.12 Trials were reported between 2002 and 2011, table 1 summarizes the characteristics of included studies.
Characteristics of RCTs reporting the effect of Acetyl-L-Carnitine for Hepatic Encephalopathy.
| Study | Year | Country | West Haven grade of HE | Sample size (ALC/placebo) | Medication (treatment/ comparison) | Duration | Outcome |
|---|---|---|---|---|---|---|---|
| Mar Malaguarnera9 | 2011 | Italy | SHE | 67 (33/34) | T: ALC 2g, bid C: placebo | 90d | ALT, AST, y-GT, ALB, ALP, PT, serum ammonia, psychometric tests, EEG, HRQL, etc. |
| Mic Malaguarnera10 | 2011 | Italy | HE 3 | 60 (30/30) | T: ALC 2g, bid C: placebo | 90d | Psychometric tests, EEG, ALT, AST, PT, serum ammonia, etc. |
| Angelo Cecere11 | 2002 | Italy | SHE | 27 (16/11) | T: LC 6g/day C: no treatment | 4 we eks | Psychometric test, serum ammonia, neurological function test, etc. |
| Mic Malaguarnera12* | 2011 | Italy | HE 1 and HE 2 | 121 (61/60) | T: ALC 2g, bid C: placebo | 90d | Mental fatigue score, serum ammonia, etc. |
| Mar Malaguarnera13 | 2008 | Italy | SHE | 125 (65/60) | T: ALC 2g, bid C: placebo | 90d | Liver Function Assessment, EEG, ALT, AST, serum ammonia, etc. |
| Mar Malaguarnera14 | 2005 | Italy | SHE | 150 (75/75) | T: ALC 2g, bid C: placebo | 90d | Liver Function Assessment, serum ammonia, etc. |
| Mar Malaguarnera15 | 2003 | Italy | HE 1 and HE 2 | 120 (60/60) | T: ALC 2g, bid C: placebo | 60d | Serum ammonia, etc. |
The quality scores of the 7 RCTs are shown in table 2. We have ensured that the 7 RCTs had high-quality.18
Jadad quality score of RCT included in the meta-analysis.
| Ref. | Randomization | Blinding | Withdrawals and dropouts | Jadad Score | Quality |
|---|---|---|---|---|---|
| 17 | With computer-generated randomization | Adequate | Clearly reported | 5 | High |
| 18 | With computer-generated randomization | Adequate | Clearly reported | 5 | High |
| 19 | Randomization mentioned, but method not specified | Not performed | Clearly reported | 2 | Low |
| 20 | With computer-generated randomization | Adequate | Clearly reported | 5 | High |
| 21 | Randomization mentioned, but method not specified | Adequate | Clearly reported | 4 | High |
| 22 | Randomization mentioned, but method not specified | Adequate | Not specifically stated | 3 | High |
| 23 | With computer-generated randomization | Mentioned, but not specified | Clearly reported | 4 | High |
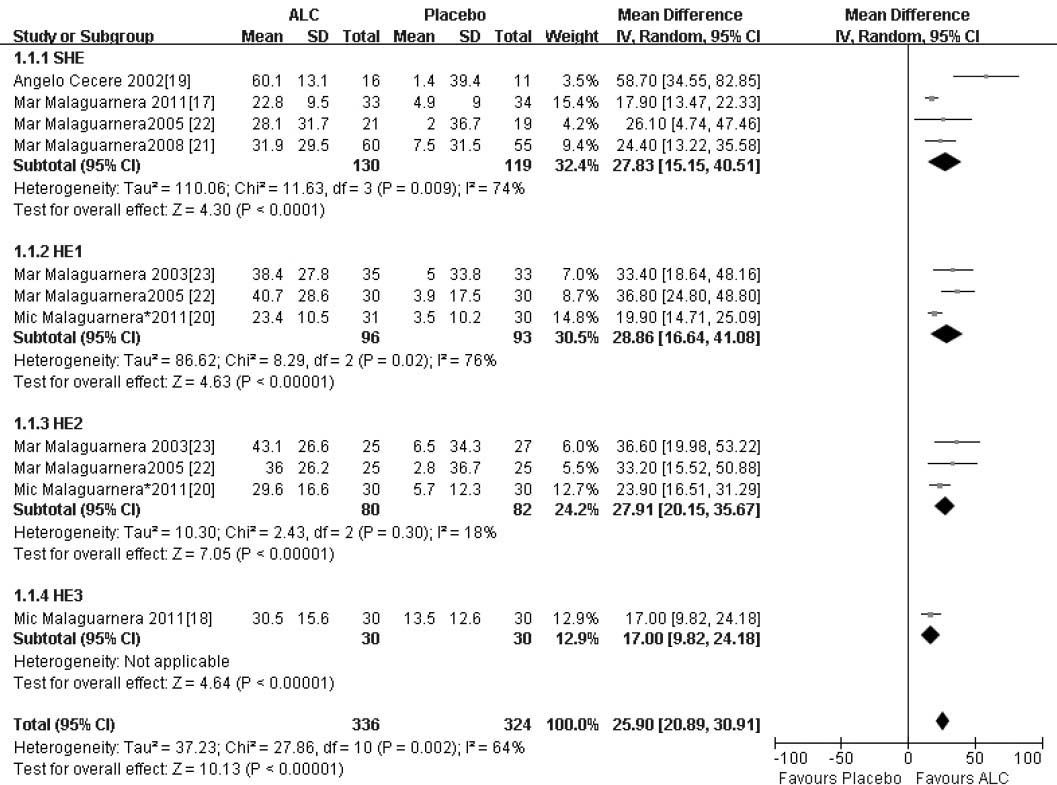
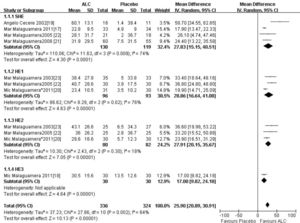
In our study, the trials consistently demonstrated an improvement in serum ammonia with ALC compared with placebo, and ALC was associated with more improved serum ammonia levels. There was significant heterogeneity among these studies (X2 = 27.86, 10 degrees of freedom, P < 0.05) and analysis by random effects modeling indicated a DL random effect WMD = 25.90 (95% CI 20.89-30.91; P < 0.05) (Figure 2).
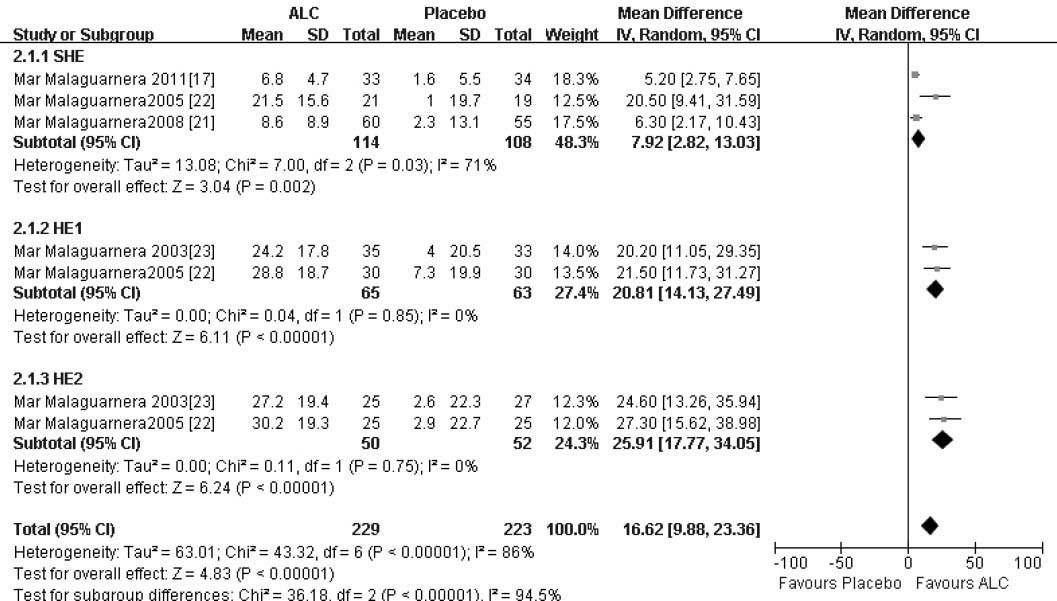
The similar outcome was manifested of NCT-A with more improved completion time in the ALC arm compared that of the placebo arm, a statistical ly significant difference. There was significant heterogeneity among these studies (X2 = 43.32, 6 degrees of freedom, P < 0.05) and analysis by random effects modeling indicated a DL random effect WMD = 16.62 (95% CI 9.88-23.36; P < 0.05) (Figure 3).
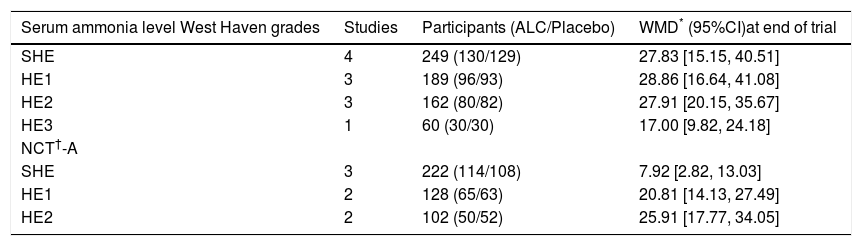
Table 3 summarized the treatment effects for patients divided into different West Haven grades.
The treatment effects for patients divided into different West Haven grade.
| Serum ammonia level West Haven grades | Studies | Participants (ALC/Placebo) | WMD* (95%CI)at end of trial |
|---|---|---|---|
| SHE | 4 | 249 (130/129) | 27.83 [15.15, 40.51] |
| HE1 | 3 | 189 (96/93) | 28.86 [16.64, 41.08] |
| HE2 | 3 | 162 (80/82) | 27.91 [20.15, 35.67] |
| HE3 | 1 | 60 (30/30) | 17.00 [9.82, 24.18] |
| NCT†-A | |||
| SHE | 3 | 222 (114/108) | 7.92 [2.82, 13.03] |
| HE1 | 2 | 128 (65/63) | 20.81 [14.13, 27.49] |
| HE2 | 2 | 102 (50/52) | 25.91 [17.77, 34.05] |
Publication bias were assessed for improvement of serum ammonia level of different HE grade of patients based on the West Haven criteria through both visual inspection of the funnel plot on the outcome of improvement of serum ammonia level and through statistical tests. And the statistical P was 0.332 for SHE, 0.092 for HE1 and 0.084 for HE2, respectively, implying no publication bias.
ALC was well tolerated and adverse events were reported infrequently and were minor.21–23,25
DiscussionHepatic encephalopathy continues to be a major clinical problem and major therapeutic breakthroughs are lacking in this area. The pathogenesis of hepatic encephalopathy is unknown, but there is general agreement that, in the majority of instances, it reflects a metabolic rather than a structural disorder of the brain. Consequently, treatment of hepatic encephalopathy has been directed primarily at reducing the production and absorption of gut-derived neurotoxins, particularly ammonia.
L-carnitine used to treat hyper-ammonia in children with urea-cycle enzyme deficiencies26 and valproate-induced hyper-ammonia.27 The protec tive effects of L-carnitine are centrally mediated by activation of metabotropic Glu receptors at the level of brain ammonia uptake and/or mitochondrial energy metabolism. However, very few studies that investigate the effects of L-carnitine in appropriate animal models of HE have been published. Clinical studies in humans with HE have only been performed by a few groups to date.
To our knowledge, there was only one report to determine the efficacy of ALC for HE.28 However, we found that it revealed some insufficiency in methodology and unreasonable data for analysis. Therefore, we doubted the validity of its results and significance of clinical practice and aimed to use the method recommended by the Cochrane Collaboration to perform a meta-analysis of RCTs with high quality. Although all RCTs were carried out in one single center, in Italy, we have ensured that the 7 RCTs had high-quality Jadad scores and no significant publication bias showed through statistical tests. Therefore, the outcome in our study has High reliability. The largest study included in our meta-analysis, which contributed the total 150 cases, gave a result that a significant therapeutic effect of carnitine was both observed in serum ammonia level and the NCT-A, which are accepted and reliable index for the assessment of improvement in patients with HE.24 Our meta-analysis of 7 methodologically sound RCTs involving 660 participants showed that ALC was effective to improve serum ammonia level (weighted mean difference 25.90, 95% confidence intervals 20.89 to 30.91, P < 0.05) and the number connection test completion time (weighted mean difference 16.62, 95% confidence intervals 9.88 to 23.36, P < 0.05). The outcome was consistent in subgroup analyses.
In addition, our study may provide useful information for subsequent clinical trials of medications in HE for a number of reasons. Firstly, our study further strengthen the evidence of well-designed RCTs of ALC in HE and introduce the view and methodology of evidence-based medicine (EBM) when compared with a previous review included 3 clinical trials reported in 2008.28 Secondly, perhaps more and more investigators from different countries will be interested and highlight the role of ALC and conduct studies with far more important findings, so as to provide another choice drug for HE.
We found consistent effects of ALC significantly reducing serum ammonia level and improving NCT in patients with West Haven grade 0, 1, 2 and 3 encephalopathy versus placebo prompting that ALC may be comparable to the current medications of HE, such as non-absorbable disaccharides, rifaximin, branched chain amino acids (BCAA) and L-ornithine-L-aspartate (LOLA).29 Furthermore, ALC appears to be more well-tolerated and safe with relatively low cost.
From a clinical standpoint, the results of this study are very encouraging but should be implemented with cautious, the majority of studies identified in our study was of small or moderate size and conducted in a single center by almost one group in Catania, Italy. Therefore, this limitation should be oriented to interpret the effectiveness of ALC for HE. Meta-analysis aims to overcome issues of power through pooling, thus increasing sample size and power.
Larger-scale, multi-center, placebo-controlled RCTs with important and conclusive outcome, for example PSE index, number of patients with improved clinical syndrome, are expected to identify the role of ALC therapy in HE. Consider-ing the encouraging results of a short-term administration of ALC in the treatment of cognitive and other neuropsychologial activities in cirrhotic patients, RCTs investigating whether these effects continue when L-carnitine treatment is stopped are required to confirm these findings.
Conflicts of InterestThe author(s) declare that they have no conflicts of interests, including financial and other relationship.
Authors’ ContributionsQian Jiang. Planning, data collection, study design and analysis, drafting the manuscript.
Gang Jiang. Conceiving of the study, participated in its design and helping to draft the manuscript.
Ke-qing Shi. Study design and statistical analysis. Hong Cai: data collection and analysis, and help to draft the manuscript.
Yi-xin Wang. Data collection and statistical analysis.
Ming-hua Zheng. Conceiving of the study, participated in its design, study supervision, obtained funding and helping to draft the manuscript.