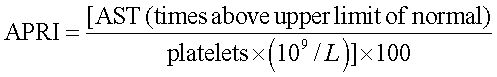Background & Aims. Variceal bleeding is a dramatic and common complication of cirrhosis, and, therefore, endoscopy is recommended for the screening of EV (esophageal varices) in every cirrhotic. This study evaluates the capacity of APRI (aspartate aminotransferase-to-platelet ratio index) in non-invasively predicting EV.
Material and methods. This cross-sectional study evaluated cirrhotics for their APRI value and the presence of EV, with a cutoff point of 1, 3; platelet count, spleen diameter, PC/SD (platelet count/ spleen diameter ratio), aspartate aminotransferase/alanine aminotransferase ratio, Child-Pugh score and MELD (model for end-stage liver disease) score were also studied.
Results. The study included 164 cirrhotics, 59.7% male, with a mean age of 56.7 years. APRI demonstrated a sensitivity of 64.7% (95% confidence interval-95%CI = 0.56-0.73), specificity of 72.7% (95%CI = 0.59-0.86), positive predictive value of 86.5% (95%CI = 0.79-0.94), negative predictive value of 43.2% (95%CI = 0.32-0.55). In the univariate analysis, platelet count, spleen diameter, Child and MELD scores, PC/SD and APRI were related to EV (p < 0.05). In the logistic regression, only platelet count and Child score were associated to EV (p < 0.05).
Conclusion. APRI is not an independent factor for the prediction of EV. Its sensitivity, specificity and predictive values are insufficient for the index to be used for the screening of EV in cirrhotics.
Variceal bleeding is the most dramatic complication of cirrhosis. The prevalence of EV (esophageal varices) in cirrhotics is between 60 and 80%, and variceal bleeding determines a mortality rate of 17% to 57%.1 Bleeding recurrence may reach 60% of patients in two years.2
Considering the impact of upper gastrointestinal bleeding due to EV rupture in the prognosis of cirrhotic patients, AASLD (American Association for the Study of Liver Disease)3 and the Baveno Consensus4 suggest that every patient diagnosed with cirrhosis should be investigated for EV. From that moment on, endoscopy will be repeatedly performed in these patients with a frequency determined by their degree of liver dysfunction and by the presence of varices and their characteristics.3,4
Primary prophylaxis against variceal bleeding is recommended mainly for medium and large varices, but also for small varices with red marks or in patients with more advanced liver disease.3–5 The indication of primary prophylaxis reinforces the need of screening cirrhotics for EV.
In order to spare patients from the discomfort and risks of endoscopy and to reduce costs, there is an effort to find non-invasive methods of screening of EV. Platelet count, spleen diameter, portal vein diameter, Child-Pugh classification, prothrombin activity, presence of telangiectasias, presence of ascites, transient elastography and a model including spider angiomas, ALT (alanine aminotransferase) and albumin, among others have been studied.6–10 Giannini, et al.1,11,12 have suggested that PC/SD (platelet count/spleen diameter ratio) could be an accurate predictor of EV in cirrhotic patients, and, as the measurement of platelets and spleen size are part of the routine workup of these patients, it could probably be the most cost-effective non-invasive method for this. While other authors did find similar results,13 we performed a study counting probably with the greatest sample including all-cause cirrhosis, performed independently from the group which first described the index and published in English and did not find satisfying results when using PC/SD for the screening of EV.14 Actually, in our study, the only variable associated to EV in the multivariate analysis was platelet count.14 Recently, 2 systematic reviews have been published on this matter, reaching contradictory conclusions,15, 16 and we believe this index is not ready to substitute endoscopy for the screening of EV.
Aspartate aminotransferase-to-platelet ratio index (APRI) was first described for the non-invasive prediction of fibrosis in patients with hepatitis C.17 After that, other authors used it in other clinical contexts and in patients with other causes of liver disease.18 Since APRI is a predictor of fibrosis, which is the major cause of portal hypertension in cirrhosis, and uses platelet count on its denominator, a variable knowingly associated to the presence of EV, it is reasonable to think that APRI could be a good non-invasive method for the screening of EV.
This study analyses the ability of APRI in predicting the existence of EV in a population of cirrhotic patients.
Material and MethodsMedical records of patients from the ambulatory care clinic of cirrhosis of Santa Casa Hospital of Porto Alegre, Brazil, were retrospectively reviewed. They should inform platelet count, AST (aspartate aminotransferase) and endoscopy 6 months apart from each other at most. Currently, endoscopy is performed in all cirrhotic patients of the hospital in the moment of the diagnosis for EV screening. EV were not classified in size for the purpose of the analyzes since even small varices are likely to bleed if presenting with red marks or in Child-Pugh class B or C patients, scenarios in which prophylaxis is already indicated.3,4
APRI was calculated as following:17
A cutoff value of 1.3 proposed by Castéra, et al. was used. Patients with an index lower than this cutoff were supposed not to have EV.19 Platelet count, spleen diameter, PC/SD (for a cutoff point of 9091,11,12), AST/ALT ratio, Child-Pugh classification and MELD (Model for End Stage Liver Disease) were also analyzed.
Data were calculated in order to verify sensitivity, specificity, negative and positive predictive values of the index, with a confidence interval of 95% and with a p value of 5%. ANOVA test was used to compare categorical and continuous variables, except when variances were not homogeneous, when Kruskal-Wallis test was used. Mantel-Haenszel’s Chi-Square test was used for comparisons between categorical variables, except values would be less than 5, when Fischer’s exact test would be preferred. APRI, platelet count, spleen diameter, PC/SD, MELD score, Child-Pugh classification and AST/ ALT were included in the univariate analysis. After the univariate analysis, variables with p < 0.1 were submitted to a logistic regression. Epi Info™ 3.4.1 was used for the statistical analysis.
It was calculated that at least 139 patients were needed to produce results with a 95% level of confidence and a 10% length for the confidence intervals. This study was approved by the Ethics Committee of Santa Casa Hospital.
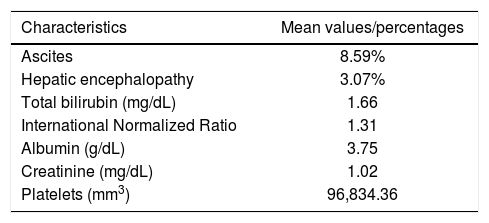
ResultsOne hundred and sixty four patients were consecutively included in the study, from which 98 were men (59.76%). The mean age of the patients was 56.70 years. Hepatitis C was present in 90 patients, and alcohol abuse, in 63 (some of the patients had both risk factors for liver disease). Patients were classified as Child-Pugh A in 86 cases (52.44%) and as Child-Pugh B or C in 78 cases (47.56%). EV were diagnosed by endoscopy in 119 patients (72.56%). Other baseline characteristics of patients are shown in table 1.
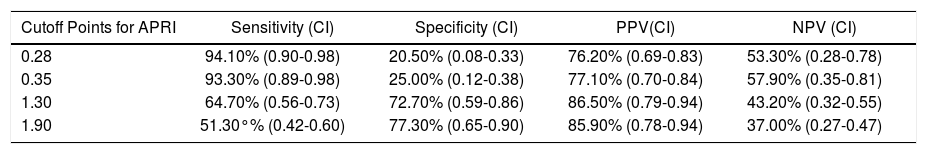
When APRI, at a cutoff of 1.3, was used in order to predict the existence of EV, it was shown a sensitivity of 64.70% (95%CI-95% confidence interval = 0.56-0.73), a specificity of 72.70% (95%CI = 0.59-0.86), a positive predictive value of 86.50% (95%CI = 0.79-0.94) and a negative predictive value of 43.20% (95%CI = 0.32-0.55). Other cutoff points were also tested, but none of them could reach a significantly better negative predictive value (Table 2).
Evaluation of different cutoff points for APRI as a predictor of esophageal varices in cirrhotic patients.
| Cutoff Points for APRI | Sensitivity (CI) | Specificity (CI) | PPV(CI) | NPV (CI) |
|---|---|---|---|---|
| 0.28 | 94.10% (0.90-0.98) | 20.50% (0.08-0.33) | 76.20% (0.69-0.83) | 53.30% (0.28-0.78) |
| 0.35 | 93.30% (0.89-0.98) | 25.00% (0.12-0.38) | 77.10% (0.70-0.84) | 57.90% (0.35-0.81) |
| 1.30 | 64.70% (0.56-0.73) | 72.70% (0.59-0.86) | 86.50% (0.79-0.94) | 43.20% (0.32-0.55) |
| 1.90 | 51.30°% (0.42-0.60) | 77.30% (0.65-0.90) | 85.90% (0.78-0.94) | 37.00% (0.27-0.47) |
APRI: aspartate aminotransferase-to-platelet ratio index. CI, 95%: Confidence Interval. PPV: positive predictive value. NPV: negative predictive value.
A subgroup analyses was carried out, evaluating only patients who had hepatitis C related cirrhosis (72 cases, after excluding patients who had association of hepatitis C and alcohol abuse). The reason for this is that APRI was first described to predict fibrosis among patients with chronic hepatitis C,17 and it could be possible that it would have a better performance among this population. In this group of patients, APRI had a sensitivity of 75.50% (95%CI = 0.64-0.87), a specificity of 63.20% (95%CI = 0.410.85), a positive predictive value of 85.10% (95%CI = 0.75-0.95) and a negative predictive value of 48.00% (95%CI = 0.28-0.68).
In the univariate analysis, APRI (p < 0.001), platelet count (p = 0.001), spleen diameter (p = 0.015), PC/SD (p < 0.001), MELD score (p = 0.017) and Child-Pugh classification (p = 0.015) were associated to the presence of EV. The only studied variable not associated to EV was the AST/ALT ratio (p = 0.512). Concerning only hepatitis C cirrhotic patients, APRI remained related to the presence of EV (p = 0.005).
When the logistic regression was performed, though, only platelet count and Child-Pugh classification could be considered independently associated to the presence of EV (p < 0.050).
DiscussionSelecting cirrhotic patients at a greater risk for EV in a non-invasive manner is of high importance in order to reduce costs, discomfort and risks related to endoscopy. Endoscopy is an invasive and expensive procedure, which could be unnecessary in a group of the patients diagnosed with cirrhosis. For a non-invasive test to be considered useful in the studied context, it should have a great negative predictive value, once misdiagnosing cirrhotic patients as not having EV is a major risk.
APRI has a good accuracy in predicting fibrosis, as it has already been shown by other authors.17,18 Platelet count is reduced in cirrhotics by different reasons: portal hypertension (through hypersplenism), antibody-mediated platelet destruction (mostly in viral hepatitis), decreased thrombopoietin production and myelotoxic effects of alcohol and hepatitis virus.20 As liver fibrosis is the major responsible for portal hypertension in cirrhosis, it would be reasonable to suppose APRI could correct thrombocytopenia for other causes which not portal hypertension and, then, be used as a good predictor of EV. Besides, this index uses two easily obtained parameters, which are part of the routine laboratory workup of cirrhotic patients and, thus, would not increase costs. Moreover, AST and platelet count are not variables predisposed to significant measure errors. On the other hand, other options of non-in-vasively predicting the existence of EV have not yet proven to be ready to be used instead of endoscopy.9 Both PC/SD15,16 and transient elastography19,21,22 provoke divergence regarding its accuracy among different authors, and while the former would have the benefit of decreasing health care costs, the latter would not. The abovementioned was the rationale to use APRI as a non-invasive method of screening of EV.
Sanyal, et al.23 were the first authors to raise the hypothesis that APRI could be related to the presence of EV. Differently from our study, they included patients without cirrhosis, and their cirrhotic population was composed only by Child A patients, which could have impaired their results regarding the capability of APRI to predict EV. Despite including only cirrhotic patients and a significant sample of decompensated cirrhotics in our study, we reached the same results as Sanyal at al., it was associated to EV in the univariate analyzes, but not in the multivariate one.
Berzigotti, et al.6 found a relation between APRI and clinically significant portal hypertension (hepatic venous gradient pressure ≥ 10 mmHg), but did not directly correlate it with EV. On the other hand, Castéra, et al.19 proposed the cutoff of 1.3 for APRI as a predictor of EV. Nevertheless, this analysis was not the primary objective of the study, only hepatitis C positive patients and classified as Child A were included in the study, and authors also found other non-invasive methods, including transient elastography, to be superior to APRI in the prediction of EV. In the present study, we had the primary objective of evaluating APRI in the context of predicting EV and we also included patients with other causes of cirrhosis and classified as Child B or C. While they found a sensitivity of 68%, a specificity of 64%, a positive predictive value of 51% and a negative predictive value of 78%, we found values of 75.50%, 63.20%, 85.10% and 48.00% respectively (considering the hepatitis C positive patients). Sensitivity and specificity values were not as different between studies as were the predictive values, which might be explained by the fact that Child A patients, the ones exclusively included in the study by Castéra et al., have EV less frequently than Child B or C patients, which were also included in our study, and the frequency of the event (existence of EV) influence the predictive values of a given diagnostic method.
Tafarel, et al.24 studied APRI at a higher cutoff point (1.64) when compared to the present study and found it to be independently associated to the presence of EV, differently from our study. Nevertheless, when we evaluated a higher cutoff value (1.9), we still could not find good results with APRI. Yet, despite showing a better negative predictive value than we did, they had lower values of sensitivity, specificity and positive predictive value and could not recommend APRI as a substitute for endoscopy in the screening of EV.
Stefanescu, et al.21 chose a cutoff point of 1.434, which is quite similar to what we used in the present study. They found a slightly superior sensitivity (66.24%) than we did, but inferior specificity and predictive values and could not significantly correlate APRI to the presence of EV.
Wang, et al.22 proposed a cutoff point of 0.77 as being the optimal one to predict EV, found a better sensitivity (71%) and negative predictive value (79%) than we did and could significantly associate it to the existence of EV. They found it to be as good as transient elastography in the prediction of EV, but could not verify a significant improvement after combining both. Differently from our study, though, they evaluated only hepatitis B patients and did not evaluate decompensated cirrhotic patients, which could justify different results.
The present study is the only we are aware of to evaluate a wide range of cutoff points. This allowed us to prove that there is no satisfactory cutoff value for APRI to be used as a predictor of EV. A screening tool, in a context of a serious situation as the presence of EV, which is responsible for the most dramatic complication of cirrhosis, variceal bleeding, must have an excellent negative predictive value in order not to miss patients who could benefit from primary prophylaxis.
The results of this study lead to the conclusion that APRI is not an appropriate substitute for endoscopy and cannot be used in the screening of EV among cirrhotic patients.
Abbreviations- •
EV: esophageal varices.
- •
AASLD: American Association for the Study of Liver Disease.
- •
ALT: alanine aminotransferase.
- •
PC/SD: platelet count/spleen diameter ratio.
- •
APRI: aspartate aminotransferase-to-platelet ratio index.
- •
AST: aspartate aminotransferase.
- •
MELD: Model For End-Stage Liver Disease.
- •
95%CI: 95% confidence interval.
None.
Conflicts of InterestNone.