Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide and can progress to non-alcoholic steatohepatitis (NASH) and, ultimately, cirrhosis. Clostridioides difficile is the most common nosocomial cause of diarrhea and is associated with worse clinical outcomes in other liver diseases, including cirrhosis, but has not been extensively evaluated in concomitant NAFLD/NASH.
Materials and MethodsWe conducted a retrospective cohort study using the National Inpatient Sample database from 2015 to 2017. Patients with a diagnosis of CDI, NAFLD, and NASH were identified using International Classification of Diseases (Tenth Revision) codes. The outcomes of our study include length of stay, hospitalization cost, mortality, and predictors of mortality.
ResultsThe CDI and NASH cohort had a higher degree of comorbidity burden and prevalence of peptic ulcer disease, congestive heart failure, diabetes mellitus, and cirrhosis. Patients with NASH and CDI had a significantly higher mortality rate compared to the CDI only cohort (mortality, 7.11 % vs. 6.36 %; P = 0.042). Patients with CDI and NASH were at increased risk for liver-related complications, acute kidney injury, and septic shock (P < 0.001) compared to patients with CDI only. Older age, intestinal complications, pneumonia, sepsis and septic shock, and liver failure conferred an increased risk of mortality among the CDI and NASH cohort.
ConclusionsPatients with NASH had a higher rate of liver-related complications, progression to septic shock, and mortality rate following CDI infection compared to the CDI only cohort.
Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum of diseases characterized by hepatic steatosis; however, it can progress to non-alcoholic steatohepatitis (NASH), which involves progression of fatty infiltration leading to hepatocyte injury, inflammation, and necrosis. NASH can eventually lead to liver cirrhosis and end-stage liver disease and is the fastest-growing indication for liver transplantation [1]. Currently, new nomenclature of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) have been chosen to replace the terms NAFLD and NASH, respectively [2].
Clostridioides difficile (C. difficile) infection (CDI) is one of the most common nosocomial infections with increasing incidence in the United States and worldwide [3]. It is associated with increased healthcare burden by extending the length of stay, ICU admissions, and overall increases in treatment and hospitalization costs [4]. Previous studies have shown that CDI have been responsible for up to $4.8 billion in excess healthcare costs annually for acute care facilities alone [5]. CDI is associated with significant mortality and morbidity, with an all-cause mortality rate of approximately 13 % to 30 % [6,7]. According to the CDC, over half a million people are infected with C. difficile annually, with approximately 29,000 deaths within one month of initial diagnosis with C. difficile. Additionally, it is estimated that 20–30 % of patients with an initial CDI will develop a recurrent infection [8]. Major risk factors for CDI include previous antibiotic use (particularly clindamycin), advanced age, recent healthcare exposure, inflammatory bowel disease, and immunosuppression [9].
CDI is associated with worse clinical outcomes in cirrhosis, particularly a higher occurrence rate, higher recurrence rate (35 % vs. 20 % non-cirrhosis), and higher mortality rate (14 % vs. 8 % non-cirrhosis) [10-12]. NAFLD is a risk factor for CDI, and recurrent CDI and is associated with worse intestinal complications [13,14]. Although NAFLD has been associated with higher incidences of CDI, there are limited studies evaluating key CDI clinical outcomes, including mortality, morbidity, complications, and hospital utilization in NAFLD/NASH patients. With the utilization of the National Inpatient Sample (NIS), we assessed the impact of NAFLD/NASH in hospitalized patients with CDI on (i) mortality, (ii) healthcare burden, (iii) complications, and (iv) predictors of mortality.
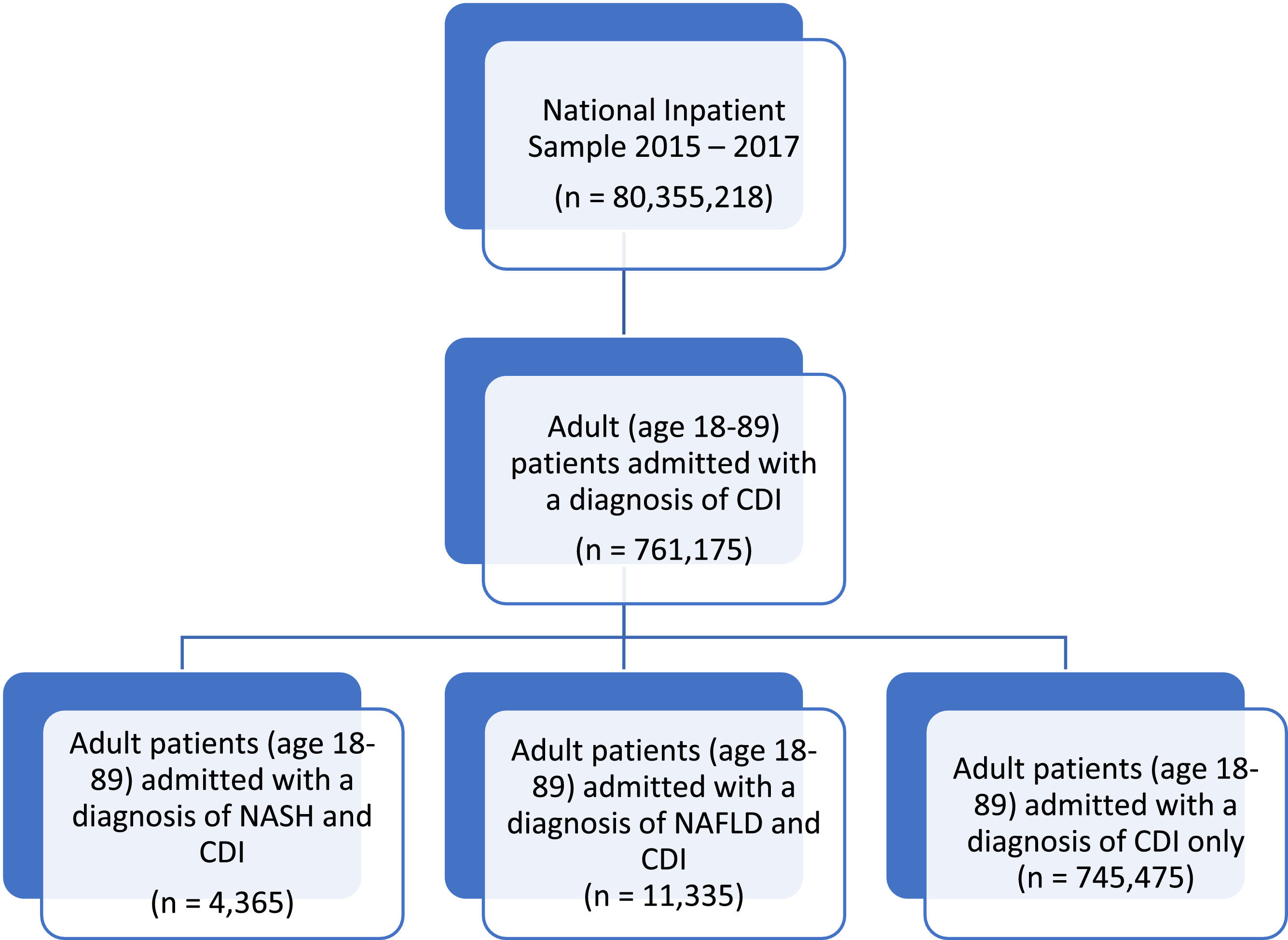
2Materials and Methods2.1Data sourceThe Healthcare Cost and Utilization Project (HCUP) is a collection of databases, including the NIS database. The NIS dataset is the largest available dataset and includes publicly available, de-identified, inpatient data from hospitalized patients across the United States. The dataset is encoded by International Classification of Diseases (ICD) codes from more than 1000 hospitals, representing a stratified sample of approximately 20 % of all hospital admissions in the United States. NIS Data does not include data from long-term acute care hospitals and rehabilitation hospitals. ICD, Tenth Revision and Clinical Modification (ICD-10-CM) codes were used to identify patients with a history of NAFLD (K76.0), NASH (K75.81), and CDI (A04.7). Primary and secondary diagnoses within the study population. International Review Board (IRB) approval was not required as the HCUP-NIS database omits patient identifiers. HCUP data use agreements were signed, submitted, and approved for data access.
2.2Study design & study populationWe performed a retrospective cohort study including patients ≥ 18 years of age using the NIS from January 1st, 2015, to December 31st, 2017. Patients with C. Difficile infection, NAFLD, and NASH were identified using ICD-10 codes. Patients with diagnoses of both NAFLD and NASH were excluded.
2.3Definition of variablesDemographic variables included age, gender, race/ethnicity, BMI, and patient's insurance. Primary outcome variables included length of stay, hospitalization costs, and inpatient mortality. A medical history comorbidity profile was obtained from each participant during the baseline period using ICD-10-CM codes. The comorbidity profile included hypertension, liver disease, cirrhosis, myocardial infarction, congestive heart failure (CHF), peripheral vascular disease (PVD), cerebral vascular accident (CVA)/transient ischemic attack (TIA), chronic obstructive pulmonary disease (COPD), connective tissue disease, diabetes mellitus (DM), peptic ulcer disease, chronic kidney disease (CKD), solid tumor, leukemia, lymphoma, and acquired immunodeficiency syndrome (AIDS). The total weighted Charlson Comorbidity Index (CCI) score was calculated for each population.
2.4OutcomesThe primary outcomes were mortality, length of stay (LOS), and hospital costs in patients with CDI only, CDI and NAFLD, or CDI and NASH. Secondary outcomes included clinical complications, namely acute kidney injury (AKI), pneumonia, respiratory failure, ventilatory dependence, acute pulmonary embolism, intestinal perforation, peritonitis, toxic megacolon, acute liver failure, liver failure, and liver cancer. Demographics, comorbidities, and complications were studied to determine mortality-associated risk factors.
2.5Statistical analysisPatient demographics, comorbidity profile, and primary and secondary outcomes were summarized using median frequencies with percentages for categorical variables or median with interquartile range for continuous variables. Bivariate analyses were completed using t-tests for normally distributed continuous variables and either Pearson's Chi-Square or Fisher exact tests for categorical variables. Normality was assessed using Shapiro-Wilk testing along with visual inspection of quantile-quantile plots. Post hoc analysis was performed using Bonferroni correction methods. Multiple logistic regression models and resulting odds ratios (ORs) [with 95 % confidence intervals (CIs)] were used to test for association between the two diagnosis groups and the outcomes of mortality further adjusting for demographics and additional clinical characteristics. All reported P-values are 2-sided and the significance cut-off was set to a value of 0.05. All analyses were completed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
2.6Ethical statementInternational Review Board (IRB) approval was not required as the HCUP-NIS database omits patient identifiers.
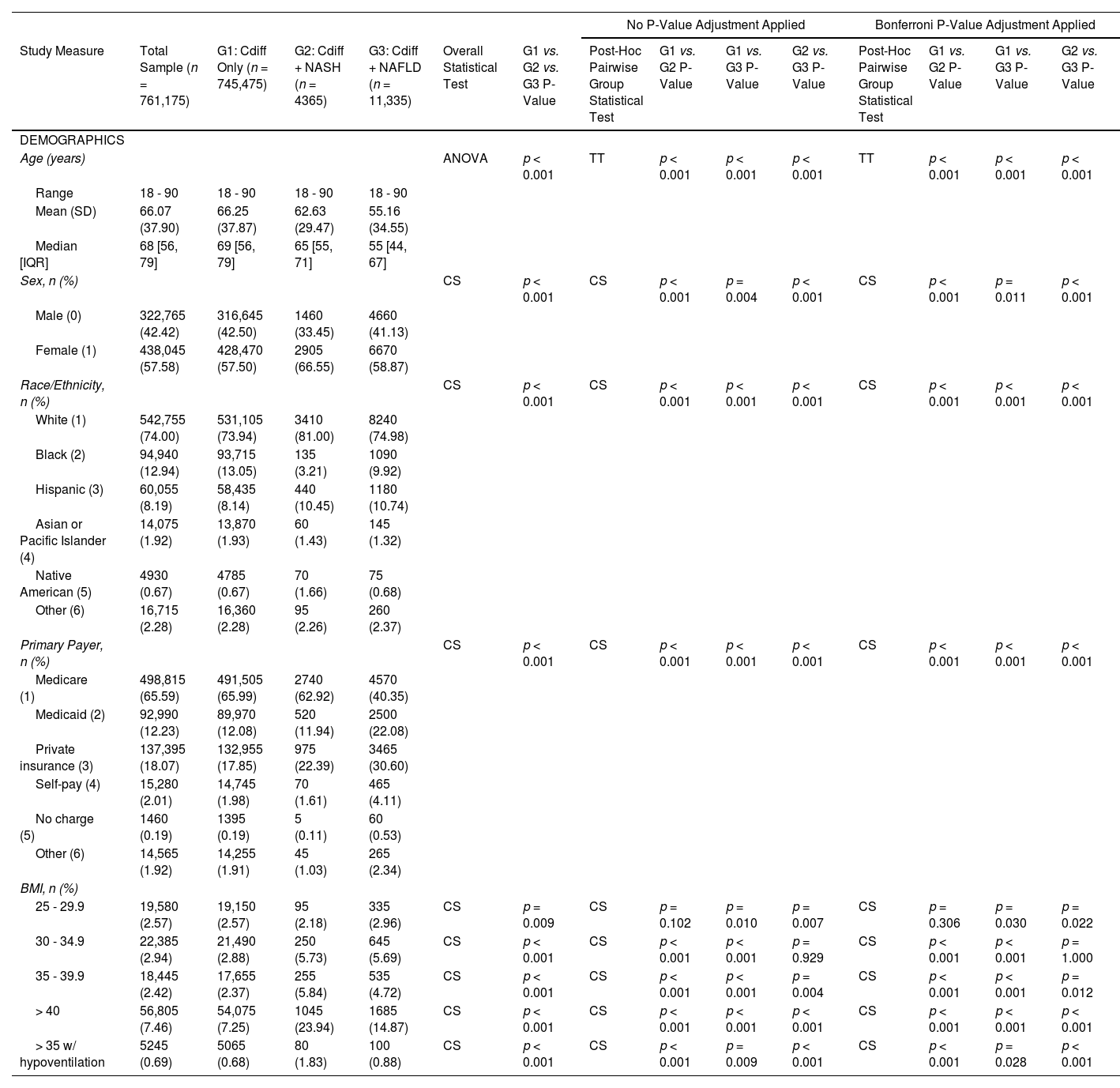
3Results3.1Sample characteristics and comorbidity profilesA total of 761,175 patients with CDI were included and, 11,335 (1.49 %) had NAFLD, and 4365 (0.57 %) had NASH (Fig. 1). Demographic and comorbidity differences between the CDI only (G1), CDI and NASH (G2), and CDI and NAFLD cohort (G3) are presented in Table 1. Compared to the total sample and CDI only cohort, CDI patients with NAFLD and NASH were younger, with a mean age of 55.16 and 62.63 years old, respectively (P < 0.001). Females and Caucasian ethnicity represent a majority of the study population, with a significantly higher proportion in the NAFLD and NASH cohorts (P < 0.05). The primary healthcare payers were Medicare and private insurance. Peptic ulcer disease, liver disease (mild, moderate to severe), cirrhosis, CHF, and diabetes mellitus were most common in the CDI and NASH cohort (P < 0.001) (Table 2). Hypertension was most common among the CDI and NAFLD cohort (P < 0.001). Patients in the CDI and NASH cohort had the greatest burden of comorbidities compared to the CDI and NAFLD and CDI only cohorts (Mean CCI, 6.33 vs. 5.12 vs. 3.40; P < 0.001).
Baseline demographics of Patients following CDI infection with and without a diagnosis of NAFLD/NASH.
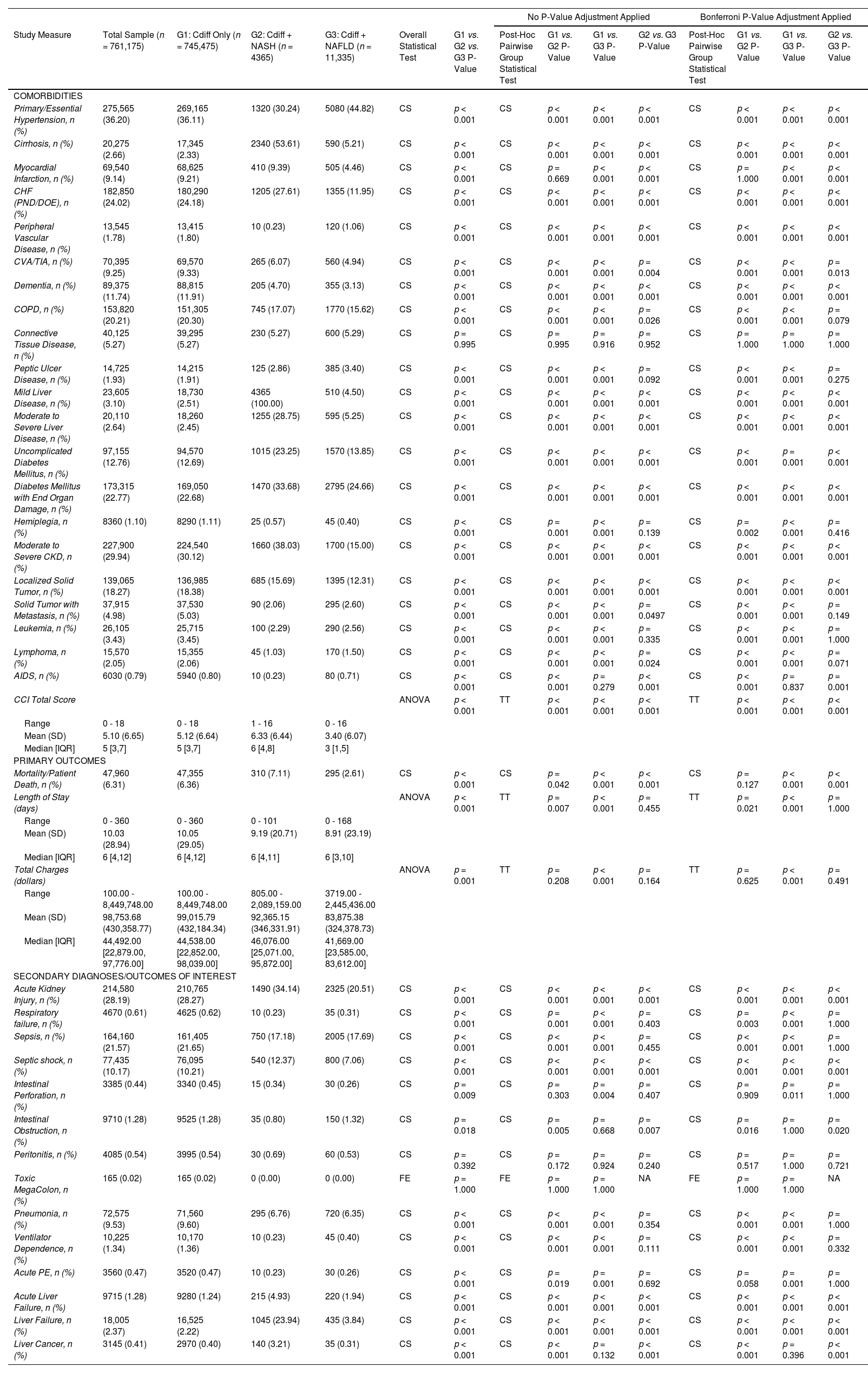
Prevalence of comorbidities and outcomes, including mortality, length of stay, and hospitalization costs, in patients with and without a diagnosis of NAFLD/NASH following CDI.
| No P-Value Adjustment Applied | Bonferroni P-Value Adjustment Applied | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Measure | Total Sample (n = 761,175) | G1: Cdiff Only (n = 745,475) | G2: Cdiff + NASH (n = 4365) | G3: Cdiff + NAFLD (n = 11,335) | Overall Statistical Test | G1 vs. G2 vs. G3 P-Value | Post-Hoc Pairwise Group Statistical Test | G1 vs. G2 P-Value | G1 vs. G3 P-Value | G2 vs. G3 P-Value | Post-Hoc Pairwise Group Statistical Test | G1 vs. G2 P-Value | G1 vs. G3 P-Value | G2 vs. G3 P-Value |
| COMORBIDITIES | ||||||||||||||
| Primary/Essential Hypertension, n (%) | 275,565 (36.20) | 269,165 (36.11) | 1320 (30.24) | 5080 (44.82) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Cirrhosis, n (%) | 20,275 (2.66) | 17,345 (2.33) | 2340 (53.61) | 590 (5.21) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Myocardial Infarction, n (%) | 69,540 (9.14) | 68,625 (9.21) | 410 (9.39) | 505 (4.46) | CS | p < 0.001 | CS | p = 0.669 | p < 0.001 | p < 0.001 | CS | p = 1.000 | p < 0.001 | p < 0.001 |
| CHF (PND/DOE), n (%) | 182,850 (24.02) | 180,290 (24.18) | 1205 (27.61) | 1355 (11.95) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Peripheral Vascular Disease, n (%) | 13,545 (1.78) | 13,415 (1.80) | 10 (0.23) | 120 (1.06) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| CVA/TIA, n (%) | 70,395 (9.25) | 69,570 (9.33) | 265 (6.07) | 560 (4.94) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p = 0.004 | CS | p < 0.001 | p < 0.001 | p = 0.013 |
| Dementia, n (%) | 89,375 (11.74) | 88,815 (11.91) | 205 (4.70) | 355 (3.13) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| COPD, n (%) | 153,820 (20.21) | 151,305 (20.30) | 745 (17.07) | 1770 (15.62) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p = 0.026 | CS | p < 0.001 | p < 0.001 | p = 0.079 |
| Connective Tissue Disease, n (%) | 40,125 (5.27) | 39,295 (5.27) | 230 (5.27) | 600 (5.29) | CS | p = 0.995 | CS | p = 0.995 | p = 0.916 | p = 0.952 | CS | p = 1.000 | p = 1.000 | p = 1.000 |
| Peptic Ulcer Disease, n (%) | 14,725 (1.93) | 14,215 (1.91) | 125 (2.86) | 385 (3.40) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p = 0.092 | CS | p < 0.001 | p < 0.001 | p = 0.275 |
| Mild Liver Disease, n (%) | 23,605 (3.10) | 18,730 (2.51) | 4365 (100.00) | 510 (4.50) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Moderate to Severe Liver Disease, n (%) | 20,110 (2.64) | 18,260 (2.45) | 1255 (28.75) | 595 (5.25) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Uncomplicated Diabetes Mellitus, n (%) | 97,155 (12.76) | 94,570 (12.69) | 1015 (23.25) | 1570 (13.85) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p = 0.001 | p < 0.001 |
| Diabetes Mellitus with End Organ Damage, n (%) | 173,315 (22.77) | 169,050 (22.68) | 1470 (33.68) | 2795 (24.66) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Hemiplegia, n (%) | 8360 (1.10) | 8290 (1.11) | 25 (0.57) | 45 (0.40) | CS | p < 0.001 | CS | p = 0.001 | p < 0.001 | p = 0.139 | CS | p = 0.002 | p < 0.001 | p = 0.416 |
| Moderate to Severe CKD, n (%) | 227,900 (29.94) | 224,540 (30.12) | 1660 (38.03) | 1700 (15.00) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Localized Solid Tumor, n (%) | 139,065 (18.27) | 136,985 (18.38) | 685 (15.69) | 1395 (12.31) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Solid Tumor with Metastasis, n (%) | 37,915 (4.98) | 37,530 (5.03) | 90 (2.06) | 295 (2.60) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p = 0.0497 | CS | p < 0.001 | p < 0.001 | p = 0.149 |
| Leukemia, n (%) | 26,105 (3.43) | 25,715 (3.45) | 100 (2.29) | 290 (2.56) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p = 0.335 | CS | p < 0.001 | p < 0.001 | p = 1.000 |
| Lymphoma, n (%) | 15,570 (2.05) | 15,355 (2.06) | 45 (1.03) | 170 (1.50) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p = 0.024 | CS | p < 0.001 | p < 0.001 | p = 0.071 |
| AIDS, n (%) | 6030 (0.79) | 5940 (0.80) | 10 (0.23) | 80 (0.71) | CS | p < 0.001 | CS | p < 0.001 | p = 0.279 | p < 0.001 | CS | p < 0.001 | p = 0.837 | p = 0.001 |
| CCI Total Score | ANOVA | p < 0.001 | TT | p < 0.001 | p < 0.001 | p < 0.001 | TT | p < 0.001 | p < 0.001 | p < 0.001 | ||||
| Range | 0 - 18 | 0 - 18 | 1 - 16 | 0 - 16 | ||||||||||
| Mean (SD) | 5.10 (6.65) | 5.12 (6.64) | 6.33 (6.44) | 3.40 (6.07) | ||||||||||
| Median [IQR] | 5 [3,7] | 5 [3,7] | 6 [4,8] | 3 [1,5] | ||||||||||
| PRIMARY OUTCOMES | ||||||||||||||
| Mortality/Patient Death, n (%) | 47,960 (6.31) | 47,355 (6.36) | 310 (7.11) | 295 (2.61) | CS | p < 0.001 | CS | p = 0.042 | p < 0.001 | p < 0.001 | CS | p = 0.127 | p < 0.001 | p < 0.001 |
| Length of Stay (days) | ANOVA | p < 0.001 | TT | p = 0.007 | p < 0.001 | p = 0.455 | TT | p = 0.021 | p < 0.001 | p = 1.000 | ||||
| Range | 0 - 360 | 0 - 360 | 0 - 101 | 0 - 168 | ||||||||||
| Mean (SD) | 10.03 (28.94) | 10.05 (29.05) | 9.19 (20.71) | 8.91 (23.19) | ||||||||||
| Median [IQR] | 6 [4,12] | 6 [4,12] | 6 [4,11] | 6 [3,10] | ||||||||||
| Total Charges (dollars) | ANOVA | p = 0.001 | TT | p = 0.208 | p < 0.001 | p = 0.164 | TT | p = 0.625 | p < 0.001 | p = 0.491 | ||||
| Range | 100.00 - 8,449,748.00 | 100.00 - 8,449,748.00 | 805.00 - 2,089,159.00 | 3719.00 - 2,445,436.00 | ||||||||||
| Mean (SD) | 98,753.68 (430,358.77) | 99,015.79 (432,184.34) | 92,365.15 (346,331.91) | 83,875.38 (324,378.73) | ||||||||||
| Median [IQR] | 44,492.00 [22,879.00, 97,776.00] | 44,538.00 [22,852.00, 98,039.00] | 46,076.00 [25,071.00, 95,872.00] | 41,669.00 [23,585.00, 83,612.00] | ||||||||||
| SECONDARY DIAGNOSES/OUTCOMES OF INTEREST | ||||||||||||||
| Acute Kidney Injury, n (%) | 214,580 (28.19) | 210,765 (28.27) | 1490 (34.14) | 2325 (20.51) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Respiratory failure, n (%) | 4670 (0.61) | 4625 (0.62) | 10 (0.23) | 35 (0.31) | CS | p < 0.001 | CS | p = 0.001 | p < 0.001 | p = 0.403 | CS | p = 0.003 | p < 0.001 | p = 1.000 |
| Sepsis, n (%) | 164,160 (21.57) | 161,405 (21.65) | 750 (17.18) | 2005 (17.69) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p = 0.455 | CS | p < 0.001 | p < 0.001 | p = 1.000 |
| Septic shock, n (%) | 77,435 (10.17) | 76,095 (10.21) | 540 (12.37) | 800 (7.06) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Intestinal Perforation, n (%) | 3385 (0.44) | 3340 (0.45) | 15 (0.34) | 30 (0.26) | CS | p = 0.009 | CS | p = 0.303 | p = 0.004 | p = 0.407 | CS | p = 0.909 | p = 0.011 | p = 1.000 |
| Intestinal Obstruction, n (%) | 9710 (1.28) | 9525 (1.28) | 35 (0.80) | 150 (1.32) | CS | p = 0.018 | CS | p = 0.005 | p = 0.668 | p = 0.007 | CS | p = 0.016 | p = 1.000 | p = 0.020 |
| Peritonitis, n (%) | 4085 (0.54) | 3995 (0.54) | 30 (0.69) | 60 (0.53) | CS | p = 0.392 | CS | p = 0.172 | p = 0.924 | p = 0.240 | CS | p = 0.517 | p = 1.000 | p = 0.721 |
| Toxic MegaColon, n (%) | 165 (0.02) | 165 (0.02) | 0 (0.00) | 0 (0.00) | FE | p = 1.000 | FE | p = 1.000 | p = 1.000 | NA | FE | p = 1.000 | p = 1.000 | NA |
| Pneumonia, n (%) | 72,575 (9.53) | 71,560 (9.60) | 295 (6.76) | 720 (6.35) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p = 0.354 | CS | p < 0.001 | p < 0.001 | p = 1.000 |
| Ventilator Dependence, n (%) | 10,225 (1.34) | 10,170 (1.36) | 10 (0.23) | 45 (0.40) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p = 0.111 | CS | p < 0.001 | p < 0.001 | p = 0.332 |
| Acute PE, n (%) | 3560 (0.47) | 3520 (0.47) | 10 (0.23) | 30 (0.26) | CS | p < 0.001 | CS | p = 0.019 | p = 0.001 | p = 0.692 | CS | p = 0.058 | p = 0.001 | p = 1.000 |
| Acute Liver Failure, n (%) | 9715 (1.28) | 9280 (1.24) | 215 (4.93) | 220 (1.94) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Liver Failure, n (%) | 18,005 (2.37) | 16,525 (2.22) | 1045 (23.94) | 435 (3.84) | CS | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 | CS | p < 0.001 | p < 0.001 | p < 0.001 |
| Liver Cancer, n (%) | 3145 (0.41) | 2970 (0.40) | 140 (3.21) | 35 (0.31) | CS | p < 0.001 | CS | p < 0.001 | p = 0.132 | p < 0.001 | CS | p < 0.001 | p = 0.396 | p < 0.001 |
Primary outcomes, including mortality, LOS, and inpatient hospitalization costs, and complications, are listed in Table 2. The CDI and NASH cohort had a significantly higher mortality rate compared to the CDI only cohort (mortality, 7.11 % vs. 6.36%; P = 0.042). LOS was higher in the CDI only cohort compared to the CDI and NAFLD/NASH cohorts. There were no significant differences in hospitalization costs between the CDI and NASH cohort and CDI only cohort. Patients with CDI and NASH were at increased risk for liver-related complications, including liver failure and liver cancer, acute kidney injury, and septic shock (P < 0.001) compared to patients with CDI only.
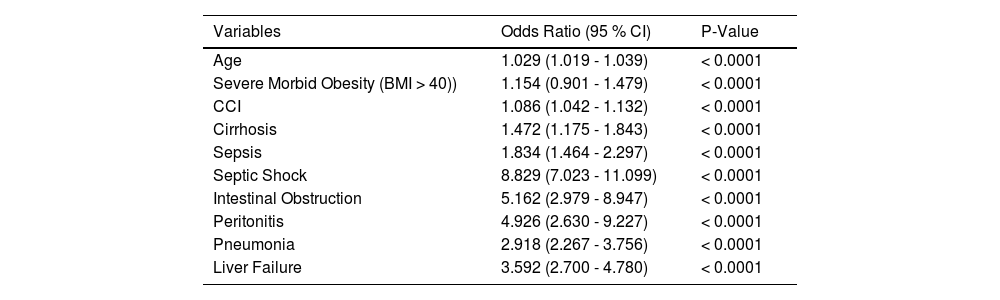
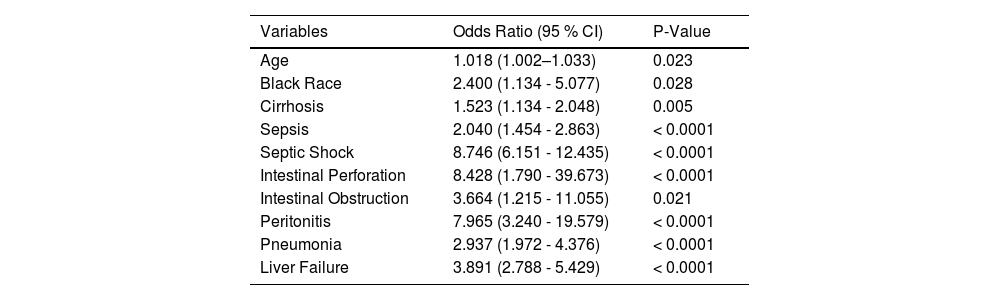
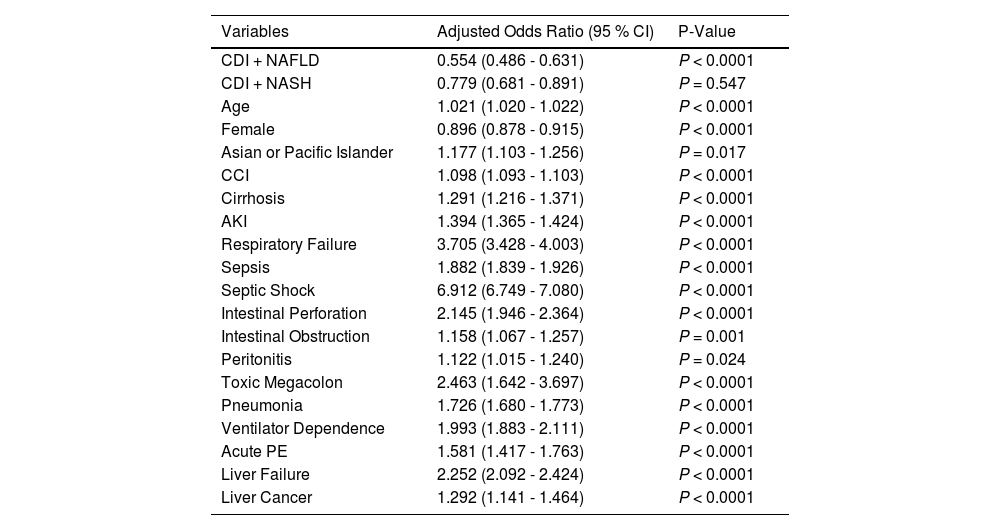
3.3Mortality risk factorsMultivariable regression analysis on death during hospitalization in patients with NAFLD/NASH with CDI is shown in Table 3. Table 4 presents predictors of mortality among the CDI and NASH cohort only. The following were shown to be predictors of mortality among the NAFLD/NASH cohort: Age (OR 1.029; P < 0.0001), Severe Morbid Obesity [BMI > 40 kg/m2] (OR 1.154; P < 0.0001), CCI (OR 1.086; P < 0.0001), Cirrhosis (OR 1.472; P < 0.001), Sepsis (OR 1.834; P < 0.0001), septic shock (OR 8.829; P < 0.0001), intestinal obstruction (OR 5.162; P < 0.0001), peritonitis (OR 4.926; P < 0.0001), and pneumonia (OR 2.918; P < 0.0001). Higher odds of mortality in the NASH cohort were shown in the following groups: Age (OR 1.023; P < 0.0001), Black ethnicity (OR 2.440; P = 0.016), Pneumonia (OR 3.069; P < 0.0001), Liver failure (OR 3.867; P < 0.0001), Intestinal obstruction (OR 5.528; P = 0.0001), Intestinal Perforation (OR 6.236; P = 0.009), Peritonitis (OR 8.044; P < 0.0001), sepsis (OR 1.926; P < 0.0001), and Septic Shock (OR 9.239; P < 0.0001). Table 5 shows an adjusted model which examines odds of inpatient mortality in patients with CDI after controlling for patient demographic characteristics and comorbidities. Based on the adjusted model, patients with co-morbid CDI with NAFLD or NASH had lower odds of inpatient mortality compared to patients with CDI only. The following were shown to be predictors of inpatient mortality among patients with CDI only in the adjusted model: Age (OR 1.021; P < 0.0001), Asian or Pacific islander (OR 1.177; P = 0.017), CCI (OR 1.098; P < 0.0001), cirrhosis (OR 1.291; P < 0.0001), AKI (OR 1.394; P < 0.0001), respiratory failure (OR 3.705; P < 0.0001), sepsis (OR 1.882; P < 0.0001), septic shock (OR 6.912; P < 0.0001), intestinal perforation (OR 2.145; P < 0.0001), intestinal obstruction (OR 1.158; P = 0.001), peritonitis (OR 1.122; P = 0.024), toxic megacolon (OR 2.463; P < 0.0001), pneumonia (OR 1.726; P < 0.0001), ventilator dependence (OR 1.993; P < 0.0001), acute PE (OR 1.581; P < 0.0001), liver failure (OR 2.252; P < 0.0001), and liver cancer (OR 1.292; P < 0.0001).
Multivariable analysis of risk factors predicting mortality among the NAFLD/NASH cohort.
Multivariable analysis of risk factors predicting mortality among the NASH cohort only.
Multivariable linear regression analysis of predictors of inpatient mortality in patients with CDI.
* Logistic regression adjusted for demographic characteristics (age, gender, race, BMI, payer/insurance) and comorbidities (hypertension, cirrhosis, acute kidney injury, respiratory failure, sepsis, septic shock, intestinal perforation, intestinal obstruction, peritonitis, toxic megacolon, pneumonia, ventilatory dependence, acute pulmonary embolism, acute liver failure, liver failure, liver cancer).
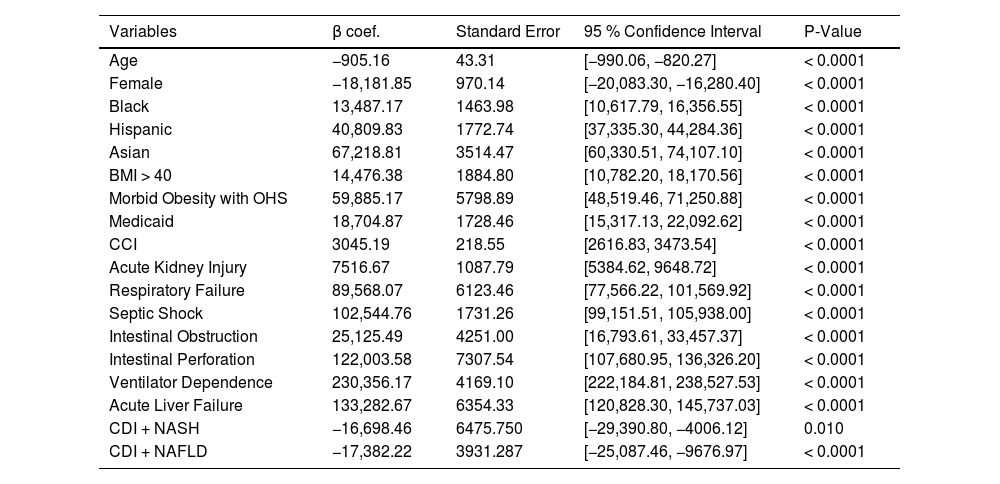
Multivariable linear regression analysis showing predictors of inpatient hospitalization costs is shown in Table 6. Black, Hispanic, and Asian race was associated with significantly higher hospitalization costs compared to White race. Patients with Obesity Class III (BMI > 40 kg/m2) and morbid obesity with obesity hypoventilation syndrome were associated with increased costs. Other predictors of increased inpatient hospitalization costs include: Medicaid insurance, CCI, AKI, respiratory failure, ventilator dependence, septic shock, intestinal obstruction, intestinal perforation, and acute liver failure. Factors that were inversely correlated with greater inpatient hospitalization costs include: age and female gender.
Multivariable linear regression analysis of predictors of inpatient hospitalization costs.
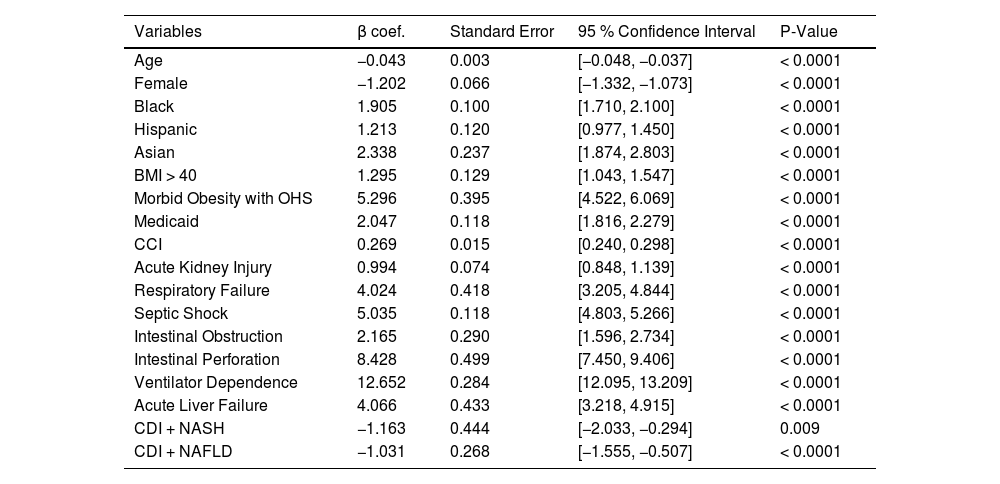
Multivariable linear regression analysis showing predictors of LOS is shown in Table 7. Age and female gender were inversely associated with LOS. Black, Hispanic, and Asian races were associated with longer LOS when compared to White races. Patients with Obesity Class III (BMI > 40 kg/m2) and morbid obesity with obesity hypoventilation syndrome were associated with longer LOS. Other predictors of increased LOS include Medicaid insurance, CCI, AKI, respiratory failure, ventilator dependence, septic shock, intestinal obstruction, intestinal perforation, and acute liver failure.
Multivariable linear regression analysis of predictors of length of stay.
Our retrospective cohort study demonstrates that there is increased mortality and liver-related complications in hospitalized patients with NASH who develop CDI when compared to those with CDI only. With approximately 30 % of the US population having NAFLD, 5 % having NASH [15] and CDI affecting approximately 13 out of every 1000 hospitalized patients[16], investigating the underlying causes behind these findings is an increasingly urgent question.
The outcomes are particularly worse for those who have NASH and CDI. The mortality rate was higher in the NASH and CDI cohort compared to CDI only cohort (mortality rate, 7.11 % vs. 6.36 %; P = 0.042). Those with NASH and CDI were also found to have higher rates of acute kidney injury, higher rates of septic shock, acute liver failure, liver failure, and liver cancer. However, after adjustment for covariates, we observed that patients with co-morbid NAFLD or NASH had lower odds of inpatient mortality compared to patients with CDI only. The CDI and NAFLD cohort had significantly lower mortality compared to the CDI only cohort (mortality rate, 2.61 % vs. 6,36 %; P < 0.001). Currently, there is a lack of data on outcomes in NAFLD patients following CDI. However, in a large retrospective study comparing patients with coexisting CDI and NAFLD to patients with coexisting alcoholic liver disease and viral liver disease, patients in the NAFLD group had lower mortality, LOS, and incidence of respiratory failure, septic shock, and renal failure [13]. In our study, it is important to note that the CDI and NAFLD cohorts were significantly younger and had a lesser degree of comorbidity burden (mean CCI, 3.40 vs. 5.12; P < 0.0001).
There are several mechanisms that may help to explain why patients with NASH and CDI have worse outcomes than those with CDI alone. One explanation is the dysregulation of the immune system in NASH. In animal models, high-fat diets lead to increased pro-inflammatory cytokine expression by hepatic macrophages [17]. This subsequently led to natural killer T cell (NKT) overactivation and apoptosis, resulting in a net NKT deficit [17]. Overactivation of hepatic macrophages also seems to play a key role in the progression of NAFLD into NASH, highlighting their inflammatory effects [18]. These findings demonstrate that NASH is a systemic disease with consequences that extend far beyond the liver.
Alterations in the gut microbiota also likely play a key role in the relationship between NASH and CDI [19]. It has been shown that patients with NASH have significantly different microbiomes when compared to obese and healthy controls, although it is not clear which alteration (progression to NASH versus changes in the gut microbiome) is first in the causal chain [20]. An increase in the prevalence of organisms such as Firmicutes and Proteobacteria, which are elevated in CDI[21], have also been found to be elevated in the gut of those with NASH [20]. Interestingly, the use of statin, which is commonly used in patients with metabolic syndrome and NAFLD/NASH, has been associated with a lower risk of recurrent CDI due to both modulation of the gut microbiome and anti-inflammatory properties [14]. These alterations may explain why recent studies have shown NAFLD to be an independent risk factor for CDI [22].
Our findings are similar to those evaluating CDI in other liver diseases. Outcomes of CDI infection with co-morbid chronic liver disease (CLD) had a higher in-hospital mortality (18.6 % vs.. 8.8 %) than those without, and patients with cirrhosis and CDI had a higher one-year mortality (9 % vs. 4 %) than CDI only patients [10]. Similarly, Bajaj et al. showed that patients with cirrhosis and CDI resulted in higher rates of mortality, LOS, and hospitalization costs compared to cirrhotic patients without CDI [23]. Kruger et al. showed higher 30-day readmission, index admission mortality, and calendar-year mortality in patients with cirrhosis and CDI compared to cirrhosis without CDI. Interestingly, the most common reason for readmission was recurrent CDI [24]. In a study using NIS, cirrhotic patients with CDI had a higher mortality rate (OR 1.47) and a higher risk of AKI (OR 2.09) [25]. Intestinal complications in NAFLD/NASH patients with CDI are also consistent with the findings from other studies, including higher rates of intestinal perforations in NAFLD and CDI compared to alcoholic liver disease and CDI (4.6 % vs. 2.2 %, p = 0.001) [13]. However, CDI with CLD is associated with a prolonged hospital stay (1.19 more days) and an average of $8632 more in total costs, while our NAFLD/NASH cohorts did not have increased LOS and hospital costs [10].
Our study has certain strengths and limitations. NIS is the largest all-payer database, including inpatient data and is representative of all regions throughout the country. Therefore, the use of NIS provides access to data from a large and diverse cohort, providing results with good external validity and are generalizable to the US population with NAFLD/NASH and hospitalized with CDI. Using a large dataset, we can generate sizeable cohorts to increase the power of our study, detect statistically significant differences between our outcomes, and more accurately assess predictors of mortality among our cohorts. However, our study has certain limitations. There are inaccuracies in capturing ICD-9 and ICD-10. Although the definition of NAFLD and NASH has become clearer in recent years, there is likely considerable incorrect use of NAFLD and NASH in patients with fatty liver disease. There may be patients in the CDI only cohort with undiagnosed NAFLD/NASH, thereby including them in the wrong cohort. Longitudinal outcomes data following discharge is not included in NIS, so we were only able to report inpatient mortality rather than the true mortality rate. In addition, NIS does not include clinical data points, such as vital signs, laboratory values, medication use, and diagnostic studies, and therefore, it could not be included to further characterize our cohort. Lastly, NIS does not capture reasons for length of stay or hospitalization cost. Therefore, we are unable to accurately assess whether these factors were influenced by factors unrelated to clinical need and, instead, driven by disposition or social issues.
5ConclusionsFew studies have examined clinical outcomes and healthcare burdens due to CDI in patients with NAFLD/NASH. Our study, albeit with limitations, demonstrates that a diagnosis with NASH may be associated with greater inpatient mortality following CDI compared to patients without NASH. Significant risk factors, such as older age, cirrhosis, and intestinal complications, confer a higher risk of death in our cohort. These findings emphasize a need for more effective strategies, including antimicrobial precautions and stewardship, in these patient populations to prevent infection or infection-related complications and improve hospitalization outcomes.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsAll authors met the four criteria for authorship as delineated in the International Committee of Medical Journal Editors (ICMJE) guidelines.