Type 2 Diabetes Mellitus (T2DM), a prevalent metabolic disorder, often coexists with a range of complications, with retinopathy being particularly common. Recent studies have shed light on a potential connection between diabetic retinopathy (DR) and hepatic fibrosis, indicating a possible shared pathophysiological foundation in T2DM. This study investigates the correlation between retinopathy and hepatic fibrosis among individuals with T2DM, as well as evaluates the diagnostic value of DR for significant hepatic fibrosis.
Materials and MethodsOur cross-sectional analysis incorporated 5413 participants from the National Health and Nutrition Examination Survey (NHANES) 2005-2008. The Fibrosis-4 score (FIB-4) classified hepatic fibrosis into different grades (F0-F4), with significant hepatic fibrosis marked as F2 or higher. Retinopathy severity was determined using retinal imaging and categorized into four levels. The analysis of variance or Chi-square tests facilitated group comparisons. Additionally, the receiver operating characteristic (ROC) analysis appraised the predictive accuracy of retinopathy for significant hepatic fibrosis in the T2DM population.
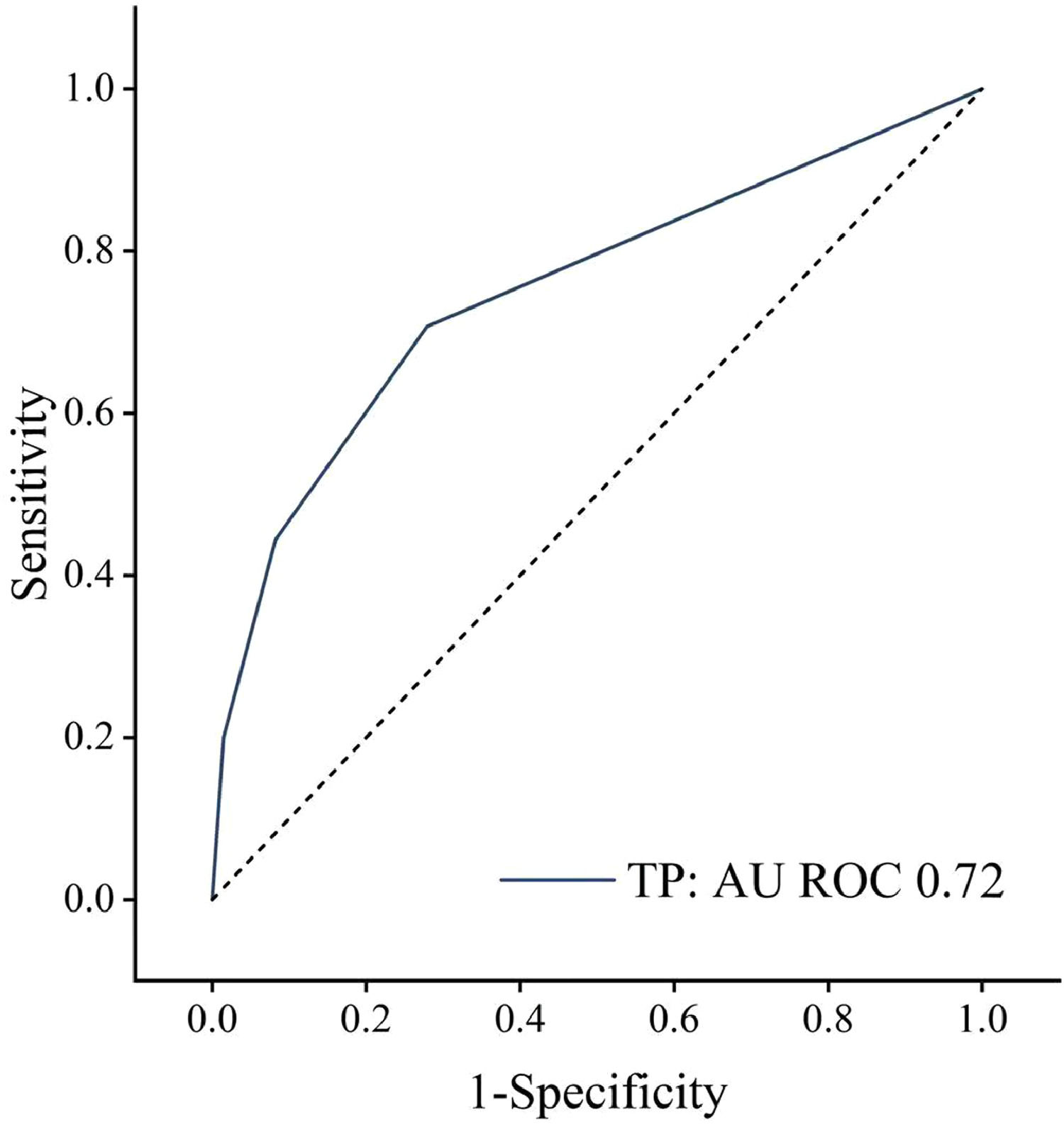
ResultsAmong 5413 participants, the mean age was 59.56 ± 12.41, with 50.2% male. And 20.6% were diagnosed with T2DM. Hepatic fibrosis grading was positively associated with retinopathy severity (OR [odds ratio]: 1.521, 95%CI [confidence interval]: 1.152-2.008, P = 0.003) across the entire population. The association was amplified in the T2DM population according to Pearson's analysis results. The ROC curve demonstrated retinopathy's diagnostic capacity for significant hepatic fibrosis in the T2DM population (AUC [area under curve] = 0.72, 95%CI: 0.651-0.793, P < 0.001).
ConclusionsRetinopathy could serve as an independent predictor of significant hepatic fibrosis in T2DM population. Ophthalmologists are advised to closely monitor T2DM patients with retinopathy.
Hepatic fibrosis often emerges as a frequent outcome of chronic hepatic injury and ranks among the significant long-term hepatic complications for patients with Type 2 diabetes mellitus (T2DM) [1,2]. Additionally, hepatic fibrosis, as a component of nonalcoholic fatty liver disease (NAFLD), has a mutual and bi-directional relationship with metabolic syndrome caused by T2DM, such as impaired glucose disposal [3]. In the absence of timely and active intervention, a fraction of fibrosis can relentlessly advance due to the synergy of cofactors, leading to severe distortion of hepatic structure. This progress may eventually result in cirrhosis and hepatocellular carcinoma, potentially leading to hepatic failure and death [4–6]. Furthermore, hepatic fibrosis has been identified as a pivotal prognostic marker of liver-related morbidity and mortality [7]. Given their intertwined pathogenesis, the combined effects of hepatic fibrosis and T2DM can exacerbate hepatic disease and amplify the risk of chronic vascular complications in diabetes [8].
Early screening for hepatic fibrosis in patients with T2DM is a vital clinical consideration. Despite hepatic biopsy being recognized as the current gold standard for assessing the severity and stage of hepatic fibrosis, its inherent risks and associated high costs limit its viability as a routine screening method [9]. Non-invasive imaging evaluations, such as ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI), offer alternative diagnostic approaches. However, they are not without limitations in terms of convenience and patient compliance [10,11]. Therefore, the need to explore an accessible, non-invasive, and affordable diagnostic tool to facilitate the early detection of hepatic fibrosis progression is paramount.
Retinopathy is a common microvascular complication of T2DM, resulting in the destruction of the blood-retinal barrier, pathological angiogenesis, and scar formation [12]. Given the established correlation between hepatic fibrosis and microvascular complications [13], it is plausible to hypothesize a potential link between diabetic retinopathy (DR) and hepatic fibrosis. However, this conclusion remains a point of contention [14].
In this light, this cross-sectional study leverages data from the National Health and Nutrition Examination Survey (NHANES) 2005-2008 to investigate the link between DR and hepatic fibrosis in the T2DM population. The study aims to employ simple, non-invasive retinopathy screening to identify T2DM individuals at risk of hepatic fibrosis, thereby providing novel insights for hepatic fibrosis screening.
2Materials and methods2.1Study population and baseline characteristicsThe NHANES, a cross-sectional population-based investigation, assesses the health and nutritional status of communities across the United States [15]. Since the 1960s and continuing since 1999, NHANES surveys approximately 5,000 nationally representative individuals annually. The resultant data, amassed within the NHANES database, is publicly accessible and instrumental in guiding medical, environmental, and public health decisions.
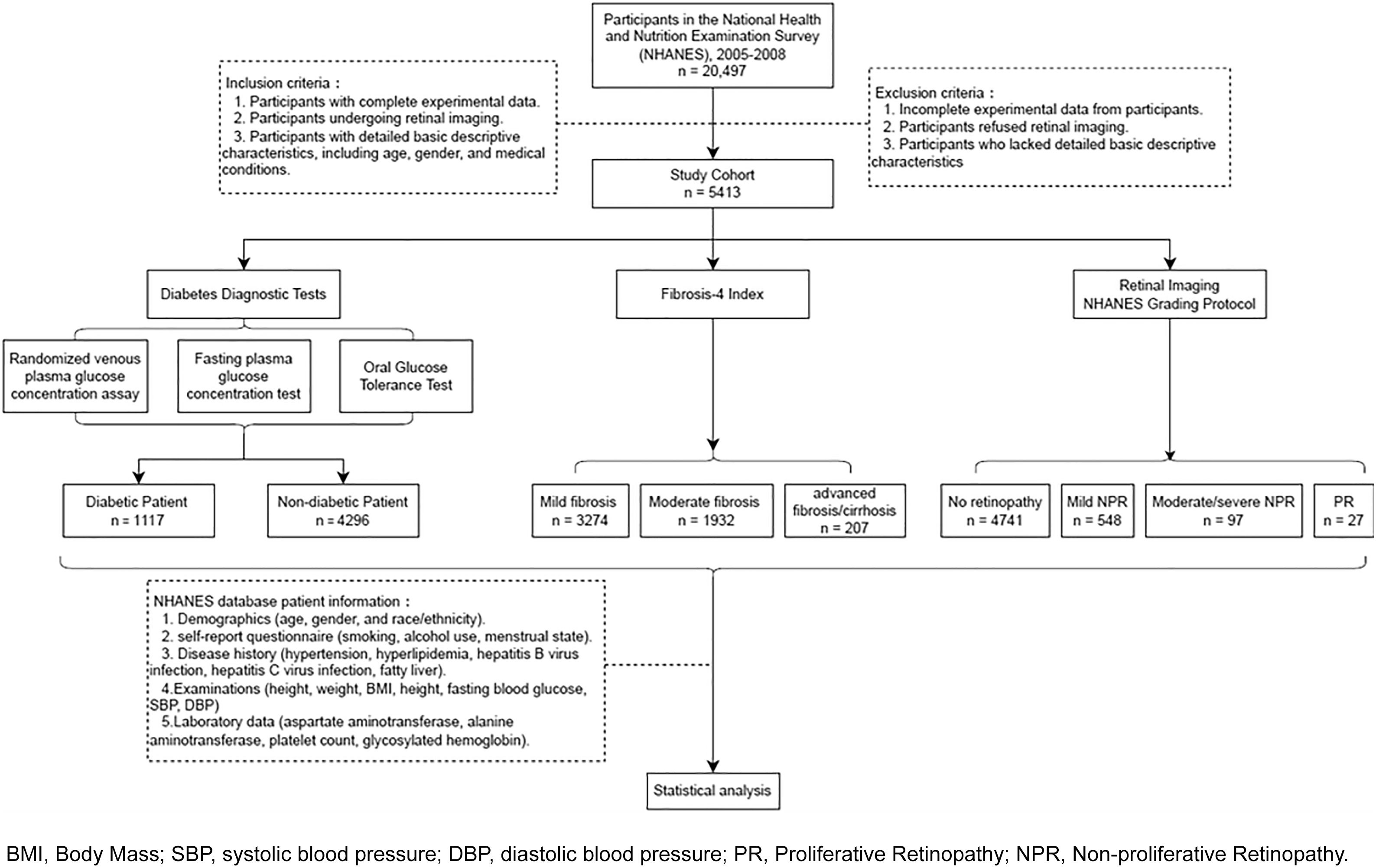
Of the 20,497 participants from the NHANES cycles of 2005 to 2008, retinal imaging was conducted for 5,704 individuals. Based on the flow chart depicted in Fig. 1, a total of 5,413 participants were included in this study.
This study analyzes demographic (age, sex, and race/ethnicity), self-reported questionnaires (smoking and drinking), history of disease (hypertension, hyperlipemia, hepatitis B virus infection, and hepatitis C virus infection), examination (height (cm), weight (kg), body mass index (BMI, kg/m2), fasting glucose (mg/dL), systolic blood pressure (SBP, mmHg), and diastolic blood pressure (DBP, mmHg)), and laboratory data (aspartate aminotransferase (AST, U/L), alanine aminotransferase (ALT, U/L), platelet count (PLT, ×10⁹/L); Glycosylated hemoglobin (HbA1c, %); and Fatty Liver Index (FLI) [16]).
2.2Diagnostic criteria for T2DMT2DM was diagnosed based on specific diagnostic criteria [17], including either a random venous plasma glucose concentration ≥ 11.1 mmol/L, a fasting plasma glucose concentration ≥ 7.0 mmol/L (whole blood ≥ 6.1 mmol/L), or a two-hour plasma glucose concentration ≥ 11.1 mmol/L following a 75g anhydrous glucose oral glucose tolerance test (OGTT). Patients who had ever told having a physician-diagnosed diagnosis of T2DM or were currently taking insulin/diabetes pills were also diagnosed with T2DM.
2.3Severity of retinopathyRetinopathy severity was assessed using retinal imaging and categorized into four levels according to the NHANES Grading Protocol [18]: Level 1 = no retinopathy; Level 2 = mild non-proliferative retinopathy (NPR); Level 3 = moderate or severe NPR; Level 4 = proliferative retinopathy (PR) with new vessels on the disc. DR was defined based on both diagnoses of retinopathy and T2DM.
2.4Grades of hepatic fibrosisHepatic fibrosis grades were determined using the Fibrosis-4 score (FIB-4). The FIB-4 has been validated in other chronic liver diseases to identify patients with significant fibrosis [19]. The FIB-4 calculation, based on age, AST, ALT, and PLT, was used to assess different hepatic fibrosis stages.
The FIB-4 calculation classifications were as follows:
FIB-4<1.45 equated to mild fibrosis (F0-F1), 1.45–3.25 to moderate fibrosis (F2), and >3.25 to advanced fibrosis/cirrhosis (F3-F4), with significant hepatic fibrosis being defined as grade F2 or higher [20].
2.5Statistical analysisStatistical analysis was conducted using SPSS software (Version 22·0) or R software (Version 3 ·3·2). Continuous variables were reported as the number of observations (n), with normally distributed data described using Mean ± Standard Deviation (SD) and non-normally distributed data characterized by the Median and Interquartile Range (IQR). Categorical variables are summarized by percentage (frequency). Significant differences between the entire population and T2DM population were estimated by chi-square test. Univariate and multivariate logistic regression analyses were performed on the association between clinical factors and hepatic fibrosis grading. The receiver operating characteristic (ROC) analysis and Pearson correlation coefficient were harnessed to evaluate the predictive efficacy of retinopathy for significant hepatic fibrosis. P <0.05 was statistically significant.
2.6Ethical statementThe collection of human tissue samples or clinical data was not involved in this study. All data was downloaded from network open databases. The NHANES protocol is approved by the National Center for Health Statistics Research Ethics Review Board, and all participants provide written informed consent.
3Results3.1Characterization of study participantsThe study cohort encompassed 5,413 individuals, with a mean age of 59.56 ± 12.41 years, and approximately half being male (50.2%). Of these, 1,117 (20.6%) individuals were diagnosed with T2DM. The T2DM subgroup was significantly older than the non-T2DM population (mean age: 63.39 ± 10.75 vs. 58.56 ± 12.62 years, P < 0.001). Compared with the non-T2DM population, T2DM population were more likely to be higher SBP (134.37 ± 20.62 vs. 128.83 ± 19.35, P < 0.001), lower DBP (69.65 ± 12.22 vs. 72.71 ± 11.61, P < 0.001), higher fasting blood glucose (8.68 ± 2.77 vs. 5.60 ± 0.37, P < 0.001), higher FLI (72.96 ± 25.63 vs. 52.82 ± 29.80, P < 0.001), and higher level of HbA1c (7.20 ± 1.69 vs. 5.50 ± 0.44, P < 0.001). In the T2DM subgroup, there were more people with a history of hypertension and hyperlipemia (66.7% (745) vs. 39.9% (1714); 52.9% (591) vs. 39.4% (1691), P < 0.001) than non-T2DM population. Within the T2DM subset, the prevalence of retinopathy was notably higher than in the non-T2DM population (30.3% vs. 11.8%, P < 0.001). Additionally, the T2DM subset exhibited greater BMI scores (32.14 ± 7.05 vs. 28.51 ± 6.05, P < 0.001) and higher FIB-4 scores (1.42 ± 0.80 vs. 1.30 ± 0.78, P < 0.001) compared to the non-T2DM population (Table 1).
Comparison of baseline characteristics between T2DM and non-T2DM population
| Characteristics | Non-T2DM population | T2DM population | χ2/t | P-Value | |
|---|---|---|---|---|---|
| (n=4296) | (n=1117) | ||||
| Age (years, x̅±s) | 58.56 ± 12.62 | 63.39 ± 10.75 | 12.884 | <0.001*** | |
| Sex [% (n)] | 0.559 | 0.455 | |||
| Male | 50.0% (2146) | 51.2% (572) | |||
| Race/Ethnicity [% (n)] | 99.244 | <0.001*** | |||
| Mexican American | 14.9% (638) | 19.8% (221) | |||
| Other Hispanic | 6.8% (292) | 7.8% (87) | |||
| Non-Hispanic White | 57.3% (2462) | 42.3% (472) | |||
| Non-Hispanic Black | 17.6% (758) | 27.7% (309) | |||
| Other Race/Multi-Racial | 3.4% (146) | 2.5% (28) | |||
| Height (cm, x̅±s) | 167.60 ± 9.90 | 166.23 ± 10.32 | 3.953 | <0.001*** | |
| Weight (kg, x̅±s) | 80.30 ± 19.18 | 89.09 ± 22.11 | 12.047 | <0.001*** | |
| BMI (kg/m2, x̅±s) | 28.51 ± 6.05 | 32.14 ± 7.01 | 15.682 | <0.001*** | |
| FIB-4 score (x̅±s) | 1.30 ± 0.78 | 1.42 ± 0.80 | 4.629 | <0.001*** | |
| PLT (109/L, x̅±s) | 267.89 ± 68.83 | 262.03 ± 73.15 | 2.416 | 0.016* | |
| ALT (U/L, x̅±s) | 25.26 ± 16.51 | 26.81 ± 29.59 | 1.692 | 0.091 | |
| AST (U/L, x̅±s) | 26.49 ± 13.80 | 26.92 ± 18.07 | 0.740 | 0.459 | |
| SBP (mmHg, x̅±s) | 128.83 ± 19.35 | 134.37 ± 20.62 | 7.527 | <0.001*** | |
| DBP (mmHg, x̅±s) | 72.71 ± 11.61 | 69.65 ± 12.22 | 6.927 | <0.001*** | |
| Glycosylated hemoglobin (HbA1c, %, x̅±s) | 5.50 ± 0.44 | 7.20 ± 1.69 | 32.981 | <0.001*** | |
| Fasting blood glucose (mmol/L, x̅±s) | 5.60 ± 0.37 | 8.68 ± 2.77 | 20.878 | <0.001*** | |
| FLI | 52.82 ± 29.80 | 72.96 ± 25.63 | 16.254 | <0.001*** | |
| Drinking history [% (n)] | 47.704 | <0.001*** | |||
| Yes | 69.1% (2969) | 58.0% (648) | |||
| No | 29.0% (1246) | 39.6% (442) | |||
| Unknown/Not reported | 1.9% (81) | 2.4% (27) | |||
| Smoking history [% (n)] | 16.680 | <0.001*** | |||
| Everyday | 18.5% (796) | 14.9% (166) | |||
| Someday | 2.5% (108) | 2.1% (23) | |||
| Not at all | 31.3% (1341) | 37.5% (419) | |||
| Unknown/Not reported | 47.7% (2051) | 45.5% (509) | |||
| Hypertension [% (n)] | 259.930 | <0.001*** | |||
| Yes | 39.9% (1714) | 66.7% (745) | |||
| No | 60.0% (2579) | 32.8% (367) | |||
| Unknown/Not reported | 0.1% (3) | 0.5% (5) | |||
| Hyperlipemia [% (n)] | 41.732 | <0.001*** | |||
| Yes | 39.4% (1691) | 52.9% (591) | |||
| No | 41.9% (1799) | 35.0% (391) | |||
| Unknown/Not reported | 18.7% (806) | 12.1% (135) | |||
| Hepatitis B virus infection [% (n)] | 2.374 | 0.123 | |||
| Yes | 7.1% (307) | 8.5% (95) | |||
| No | 92.8% (3988) | 91.5% (1022) | |||
| Unknown/Not reported | 0.1 (1) | ||||
| Hepatitis C virus infection [% (n)] | 6.600 | 0.011* | |||
| Yes | 3.0% (128) | 1.5% (17) | |||
| No | 96.9% (4164) | 98.1% (1095) | |||
| Unknown/Not reported | 0.1% (4) | 0.4% (5) | |||
| Retinopathy severity [% (n)] | 278.868 | <0.001*** | |||
| No retinopathy (Level 1) | 88.2% (3789) | 69.7% (779) | |||
| Mild NPR (Level 2) | 9.8% (421) | 19.9% (222) | |||
| Moderate/severe NPR (Level 3) | 1.4% (61) | 7.7% (86) | |||
| PR (Level 4) | 0.6% (25) | 2.7% (30) | |||
| Hepatic fibrosis grading [ % (n)] | 31.860 | <0.001*** | |||
| F0-F1 | 63.9% (2747) | 53.2% (594) | |||
| F2 | 32.7% (1404) | 40.7% (455) | |||
| F3-F4 | 3.40% (145) | 6.1% (68) | |||
T2DM, diabetes mellitus; BMI, body mass index; FIB-4, fibrosis index-4; PLT, platelet count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SBP, systolic blood pressure; DBP, diastolic blood pressure; FLI, fatty liver index; NPR, non-proliferative retinopathy; PR, proliferative retinopathy.
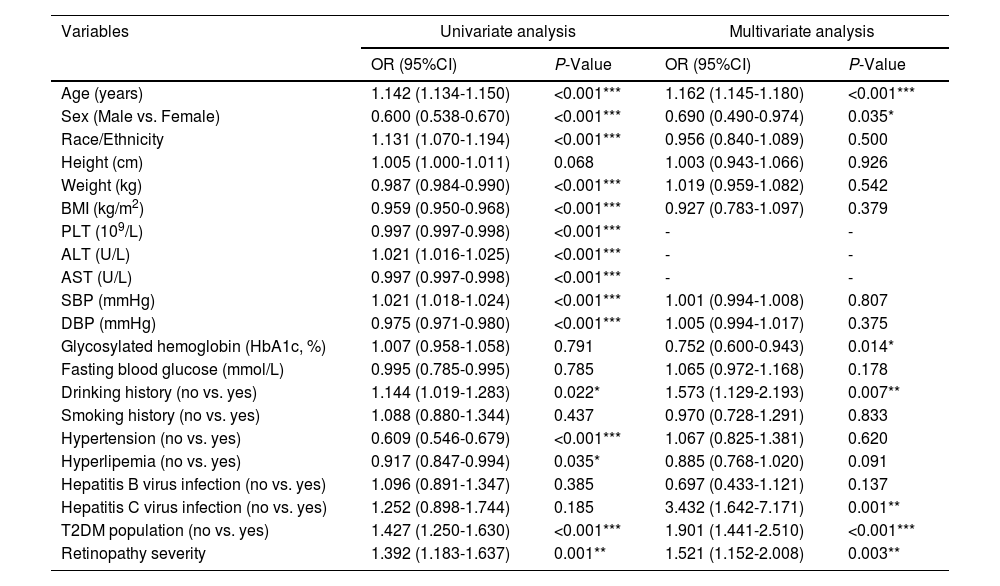
Table 2 showed that age (OR: 1.162, 95%CI: 1.145-1.180, P < 0.001), sex (OR: 0.690, 95%CI: 0.490-0.974, P = 0.035), HbA1c (OR: 0.752, 95%CI: 0.600-0.943, P = 0.014), drinking history (OR: 1.573 0.752, 95%CI: 1.129-2.193, P = 0.007), hepatitis C virus infection (OR: 3.432, 95%CI: 1.642-7.171, P = 0.001), T2DM population (OR: 1.901, 95%CI: 1.441-2.510, P < 0.001) and retinopathy severity (OR: 1.521, 95%CI: 1.152-2.008, P = 0.003) were clinical factors associated with hepatic fibrosis grading after fully adjusting for relevant variables (including age, sex, race/ethnicity, HbA1c, drinking history, hypertension, hyperlipemia, Hepatitis C virus infection, T2DM population, and retinopathy severity).
Univariate and multivariate logistic regression analysis of clinical factors affecting hepatic fibrosis grading
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95%CI) | P-Value | OR (95%CI) | P-Value | |
| Age (years) | 1.142 (1.134-1.150) | <0.001*** | 1.162 (1.145-1.180) | <0.001*** |
| Sex (Male vs. Female) | 0.600 (0.538-0.670) | <0.001*** | 0.690 (0.490-0.974) | 0.035* |
| Race/Ethnicity | 1.131 (1.070-1.194) | <0.001*** | 0.956 (0.840-1.089) | 0.500 |
| Height (cm) | 1.005 (1.000-1.011) | 0.068 | 1.003 (0.943-1.066) | 0.926 |
| Weight (kg) | 0.987 (0.984-0.990) | <0.001*** | 1.019 (0.959-1.082) | 0.542 |
| BMI (kg/m2) | 0.959 (0.950-0.968) | <0.001*** | 0.927 (0.783-1.097) | 0.379 |
| PLT (109/L) | 0.997 (0.997-0.998) | <0.001*** | - | - |
| ALT (U/L) | 1.021 (1.016-1.025) | <0.001*** | - | - |
| AST (U/L) | 0.997 (0.997-0.998) | <0.001*** | - | - |
| SBP (mmHg) | 1.021 (1.018-1.024) | <0.001*** | 1.001 (0.994-1.008) | 0.807 |
| DBP (mmHg) | 0.975 (0.971-0.980) | <0.001*** | 1.005 (0.994-1.017) | 0.375 |
| Glycosylated hemoglobin (HbA1c, %) | 1.007 (0.958-1.058) | 0.791 | 0.752 (0.600-0.943) | 0.014* |
| Fasting blood glucose (mmol/L) | 0.995 (0.785-0.995) | 0.785 | 1.065 (0.972-1.168) | 0.178 |
| Drinking history (no vs. yes) | 1.144 (1.019-1.283) | 0.022* | 1.573 (1.129-2.193) | 0.007** |
| Smoking history (no vs. yes) | 1.088 (0.880-1.344) | 0.437 | 0.970 (0.728-1.291) | 0.833 |
| Hypertension (no vs. yes) | 0.609 (0.546-0.679) | <0.001*** | 1.067 (0.825-1.381) | 0.620 |
| Hyperlipemia (no vs. yes) | 0.917 (0.847-0.994) | 0.035* | 0.885 (0.768-1.020) | 0.091 |
| Hepatitis B virus infection (no vs. yes) | 1.096 (0.891-1.347) | 0.385 | 0.697 (0.433-1.121) | 0.137 |
| Hepatitis C virus infection (no vs. yes) | 1.252 (0.898-1.744) | 0.185 | 3.432 (1.642-7.171) | 0.001** |
| T2DM population (no vs. yes) | 1.427 (1.250-1.630) | <0.001*** | 1.901 (1.441-2.510) | <0.001*** |
| Retinopathy severity | 1.392 (1.183-1.637) | 0.001** | 1.521 (1.152-2.008) | 0.003** |
OR, odds ratio; CI, confidence interval; T2DM, diabetes mellitus; BMI, body mass index; PLT, platelet count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Subsequently, we probed the relationship between the onset and advancement of retinopathy and hepatic fibrosis among the 5413 participants sourced from the NHANES database. As hepatic fibrosis evolved and intensified, there was a concomitant increase in both the likelihood and severity of retinopathy. In patients with mild NPR (Level 2), patients with hepatic fibrosis grading F0-F1, F2, and F3-F4 accounted for 12.0%, 11.5%, and 13.6%, respectively. Among patients with moderate/severe NPR (Level 3), patients with hepatic fibrosis grades of F0-F1, F2, and F3-F4 accounted for 2.5%, 2.5%, and 8.6%, respectively. The ratios of hepatic fibrosis grades F0-F1, F2, and F3-F4 were 0.7%, 1.0%, and 5.2% in patients with PR (Level 4). In other words, a significant proportion (84.8%) of the F0-F1 group exhibited no retinopathy. This proportion precipitously decreased within populations presenting mild NPR, moderate/severe NPR, and PR, with respective frequencies of 12.0%, 2.5%, and 0.7%. As highlighted in Table 3, there is a significant positive correlation between hepatic fibrosis grading and retinopathy severity in the entire population (P < 0.001).
Chi-square test and Pearson correlation analysis between the grades of retinopathy severity and hepatic fibrosis in the entire population
| Variables | Hepatic fibrosis grading (Entire population) | χ2 | P-Value | R2 | P-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| F0-F1 | F2 | F3-F4 | |||||||
| (n=3341) | (n=1859) | (n=213) | |||||||
| Retinopathy severity [% (n)] | 69.634 | <0.001*** | 0.060 | <0.001*** | |||||
| No retinopathy (Level 1) | 84.8% (2834) | 84.9% (1579) | 72.8% (155) | ||||||
| Mild NPR (Level 2) | 12.0% (400) | 11.5% (214) | 13.6% (29) | ||||||
| Moderate/severe NPR (Level 3) | 2.5% (82) | 2.5% (47) | 8.5% (18) | ||||||
| PR (Level 4) | 0.7% (25) | 1.0% (19) | 5.2% (11) | ||||||
NPR, non-proliferative retinopathy; PR, proliferative retinopathy.
The association between the grading of retinopathy and hepatic fibrosis exhibited heightened significance in the T2DM population, as suggested by Chi-square testing and Pearson correlation analysis (P < 0.001, Table 4). The correlation coefficient was found to be 0.216. The predictive capacity of retinopathy in determining hepatic fibrosis severity was assessed through the ROC analysis. The diagnostic efficiency of retinopathy (≥Level 2) was found to be most substantial for diagnosing significant hepatic fibrosis (grade of F2 or higher). The area under the ROC curve was computed as 0.722, indicating the diagnostic efficacy for significant hepatic fibrosis upon the occurrence of retinopathy (P < 0.001, Fig. 2).
Chi-square test and Pearson correlation analysis between the grades of retinopathy and hepatic fibrosis in T2DM population
| Variables | Hepatic fibrosis grading (T2DM population) | χ2 | P-Value | R2 | P-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| F0-F1 | F2 | F3-F4 | |||||||
| (n=594) | (n=455) | (n=68) | |||||||
| Retinopathy severity [% (n)] | 104.866 | <0.001*** | 0.216 | <0.001*** | |||||
| No retinopathy (Level 1) | 73.1% (434) | 70.8% (322) | 33.8% (23) | ||||||
| Mild NPR (Level 2) | 20.0% (119) | 18.7% (85) | 26.5% (18) | ||||||
| Moderate/severe NPR (Level 3) | 6.9% (41) | 6.4% (29) | 23.5% (16) | ||||||
| PR (Level 4) | 0.0% (0) | 4.2% (19) | 16.2% (11) | ||||||
T2DM, diabetes mellitus; NPR, non-proliferative retinopathy; PR, proliferative retinopathy.
The primary aim of this study was to explore the relationship between DR and hepatic fibrosis among people with T2DM. The research subjects were mainly adults living in the United States and they filled in key questions. In our study, DR severity was observed to have a positive correlation with hepatic fibrosis. As pervasive global health concerns, T2DM and hepatic fibrosis typically manifest subtly, with a significant percentage of patients showing no clear symptoms [21–23]. Without intervention, the intertwined pathophysiology of these conditions exacerbates hepatic damage in T2DM patients, thereby increasing the risks of cirrhosis and carcinoma [24]. Thus, there's an urgent need for an easily applicable, non-invasive method for early hepatic fibrosis detection in T2DM. Our study leverages adult data from the NHANES database to investigate the correlation between retinopathy and hepatic fibrosis, indicating that the presence of retinopathy may reflect the progression of hepatic fibrosis in the T2DM population.
Retinopathy, a common chronic microvascular complication of diabetes mellitus [25], involves gradual changes in retinal microvasculature [26]. Our findings align with previous research, demonstrating a significant relationship between retinopathy and hepatic fibrosis [27], notably more pronounced in diabetic patients. Previous studies have identified associations between hepatic conditions like NAFLD and retinal vessel anomalies [28]. A potential connection could be the Liver X Receptor, involved in lipid metabolism, glucose homeostasis and inflammation [29], potentially influencing susceptibility to hepatic fibrosis [30]. Insulin resistance, dyslipidemia, endothelial dysfunction, metabolic inflammation from glucose, and lipid metabolic imbalances, and oxidative stress are suggested as shared mechanisms of retinopathy in patients with diabetes and hepatic diseases [31,32].
Further, the synergistic interaction between hepatic fibrosis and diabetes has the potential to exacerbate systemic insulin resistance and hyperglycemia [32], precipitate dyslipidemia and incite the synthesis of multiple proinflammatory mediators, culminating in chronic vascular complications in diabetes [8,33] and retinopathy progression [34].
Notwithstanding the robust sample size of this study, which supports our hypothesis, the present investigation is not without limitations that warrant consideration. Firstly, given the cross-sectional study design, the causal relationship between hepatic fibrosis and retinopathy remains to be elucidated. Secondly, this study relied solely on serological markers to construct a model for gauging the severity of hepatic fibrosis without the benefit of biopsy or Fibroscan diagnostics. Thirdly, Patients with T2DM not only have a higher age but also experience an increase in Alpha 2-macroglobulin levels due to the condition itself, both of which may impact the results of FIB-4. Additionally, due to the constraints of the NHANES database, the DM population could not be subdivided into specific categories (such as type 1, type 2, and other types). Lastly, the structural similarities between the kidneys and the eyes suggest that diseases in these two organs may share common pathogenic mechanisms [35]. However, considering the incomplete data in the NHANES database, we were currently unable to analyze the relationship between retinopathy and kidney disease. The biological rationale between the retina and kidney disease underscores the importance of further exploration in future research. Further prospective research is warranted to explore the potential causal relationship between retinopathy and the onset of hepatic fibrosis among the T2DM population. We will also explore the possibility of incorporating additional analyses using other validated non-invasive tools for fibrosis.
5ConclusionsIn conclusion, our research establishes retinopathy as an independent predictor of significant hepatic fibrosis in the T2DM population based on data from the NHANES database. This finding underscores the critical role of DR in identifying individuals at elevated risk for hepatic fibrosis, prompting ophthalmologists to closely monitor T2DM patients. However, the results do not seem to be definitive for a specific target population and should be interpreted cautiously.
Author contributionsStudy conception: Xiaolong Qi, Chuan Liu; data acquisition: Jinze Li, Yi Xiang, Chuan Liu, Jiahao Han, Ruiying Wang, Zihe Dong, Huihui Chen, Ruixia Gao; statistical analysis: Jiahao Han, Yi Xiang; drafting the initial manuscript: Jinze Li, Yi Xiang, Xiaolong Qi; critical review of the manuscript: Xiaolong Qi, Gao-Jun-Teng, Jinze Li, Yi Xiang, Youfang Gao. All authors reviewed and approved the final version of the manuscript.
FundingThe study was supported by National Natural Science Foundation of China (81827805, 82130060, 61821002), National Key Research and Development Program (2018YFA0704100, 2018YFA0704104), Jiangsu Provincial Medical Innovation Center (CXZX202219), Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions and Nanjing Life Health Science and Technology Project (202205045), Changjiang Scholars Talent Cultivation Project of Zhongda Hospital of Southeast University (2023YJXYYRCPY03), the Fundamental Research Fund of Southeast University (3290002303A2), the Key Research and Development Program of Jiangsu Province (BE2023767), Research Personnel Cultivation Programme of Zhongda Hospital Southeast University (CZXM-GSP-RC125).
The authors acknowledge all the clinical and research staff from the research centers.