Background. Hepatitis C virus (HCV) infection usually results in long-term viremia. Entry of HCV into the he-patocyte requires claudin-1, -6, -9 and occludin. The efficacy of Pegylated interferon-α (PEG-IFN) treatment against HCV infection increased when ribavirin (RBV) was added to the therapeutic scheme. Our aim was to investigate if PEG-IFN plus RBV regulate claudin expression.
Material and methods. HepG2, Huh-7 and Huh-7.5 cells were treated with PEG-IFN-α2a or α2b and/or RBV at different times before obtaining the cytosolic, membrane and cytoskeletal fractions. Claudin-1, 3, 4, 6, and 9, E-cadherin and occludin expression was evaluated by Western blot analysis. Transepithelial electrical resistance (TER) was also determined.
Results. Claudin-1, 3, 4, 6, E-cadherin and occludin are constitutively expressed mainly in HepG2 cell membrane. Claudin-1 and E-cadherin cell membrane expression diminished after exposure to PEGIFNα2b (50 ng) + RBV(50 μg); the maximal decrease was observed with 200 ng of PEG-IFNα2b + 200 μg of RBV. The effect was less intense with PEG-IFNα2a. The inhibition of claudin-1 and E-cadherin expression in Huh-7 and Huh-7.5 cells was only observed with 200 ng of PEG-IFNα2b + 200 μg of RBV. TER diminished marginally in the HCV containing hepatoma cells with 200 ng of PEG-IFNα2b + 200 ug of RBV. Claudin-1 mRNA expression level was not affected by the combined treatment.
Conclusion. The increased therapeutic efficacy of the PEG-IFNα2b plus RBV treatment could be secondary to the inhibition of claudin-1 and E-cadherin cell membrane expression.
Hepatitis C virus (HCV) results in long-term persistence of viremia in approximately 80% of cases; 20% of them develop liver cirrhosis and the remaining 5% develop hepatocellular carcinoma.1,2 HCV is a small, single strand RNA enveloped virus; its genome encodes a precursor polyprotein, which is cleaved into mature proteins, including envelope glycoproteins3 and structural proteins that promote alterations of the tight junction-associated proteins.4 Entry of HCV is dependent on claudin-1, a member of a family of 24 proteins that are essential for the paracellular pathways at tight junction strands.5 Claudin-1 is important for mediating the fusion between the viral and cellular lipid membranes.6,7 Claudin-6, claudin-9, and occludin expressed in the liver also function as additional coreceptors for hepatitis C virus.8–10 The hepatocyte CD81 tetraspanin, LDL receptor and HDL scavenger receptor class B member I also bind viral envelope glycoprotein E2 thus enhancing HCV entry in a high-density-lipoprotein-dependent manner.11
Interferons have been considered the backbone of therapy in patients with HCV infection. However the percentage of HCV-infected patients with a sustained virological response (SVR) to type I pegylated interferon increased from 40% to 50-80% when ribavirin was added to the treatment.12 The aim of our work was to evaluate if such treatment could alter the expression of claudins. We discovered that the combination of pegylated interferon (PEGF-IFN) and ribavirin (RBV) inhibited the membrane expression of claudins-1, -4 and -6 and completely abolished E-cadherin expression in HepG2 cells. Claudin-1 expression was also inhibited in Huh-7.0 and Huh-7.5 cells. Pegylated α2a and α2b interferons exerted different inhibitory activity. The transepithelial electrical resistance and claudin-1 mRNA expression was not affected by PEG-IFN-α + ribavirin treatment.
Material and MethodsCell cultureThe human hepatoma cell lines HepG2 (HB-8065 ATCC), Huh-7 and Huh-7.5 (containing subgenomic and genomic HCV RNA, respectively),13,14 were grown in Dulbecco’s modified Eagle’s medium (supplemented with 0.1 U/mL of insulin, 2 mM sodium pyruvate, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1% non-essential amino acids and 10% of heat inactivated fetal calf serum), at 37 oC in a humidified atmosphere containing 5% CO2. Cells were plated at 7 × 105 cells/dish and grown at 80-90% confluence. Experiments were performed with different concentrations and exposure times of PEG-IFNα-2a (Pegytron, Roche) or PEG-IFNα-2b (Pegasys, Schering Plough) (10-200 ng/mL) and Ribavirin (R-9644, Sigma-Aldrich Co., St. Louis, MO)(10-200 μg/mL), either alone or in combination with either PEG-IFNs.
Subcellular fractionationCells, seeded at a 7 × 105 density were grown in sterile Petri dishes and maintained with supplemented DMEM medium until confluence. Cells were incubated over night with DMEM without fetal calf serum before exposing them for 4 or 8 h to 10, 50, 100 and 200 ng/mL of either PEG-IFNs-α2 and 10, 50, 100 and 200 µg/mL ribavirin either alone or in combination. At the end of the incubation time, cells were rinsed three times with cold PBS and flash frozen at − 70 °C. The method described by Mullin, et al.15 was used to obtain different cell fractions. Cells were thawed and scraped in 1 mL of buffer A (20 mM Tris, 0.25 M Dextrose, 10 mM EGTA, 2 mM EDTA, 12 µg/mL leupeptin, 1 mg/mL trypsin inhibitor and 1:100 dilution of Sigma phosphatases inhibitor cocktail 1 and cocktail 2) at 4 oC. Afterwards the cells were sonicated for 1 min and centrifuged at 39,000 rpm for 30 min at 4 oC using a Beckman Ti 70 rotor. The supernatant (cytosolic fraction) was separated and the pellet was incubated again with buffer A supplemented with 1% Triton X-100 and centrifuged at 39,000 rpm for 30 min as above. The supernatant (membrane fraction) was removed by pipetting and the remaining pellet was incubated with lysis buffer (150 mM NaCl, 50 mM Tris-HCl, 1 mM EGTA, 1% NP-40, 0.1% deoxycholate, 0.1% SDS, 2 Lg/mL aprotinin, 2 µg/mL leupeptin, 50 µM PMSF) and centrifuged at 39,000 rpm for 30 m. At the end the supernatant (cytoskeletal fraction) was removed by pipetting. All fractions were immediately frozen at −70 oC until use.
Western blot analysisWestern blot analysis was performed using the subcellular fractions. Protein concentration of each fraction was determined with a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). Fifty µg of protein was separated by 12% SDS-PAGE on a BioRad Protean II electrophoresis apparatus. Protein transfer to 0.45-micron nitrocellulose membrane (Bio-Rad) was performed at 120 mA for 1 h using a Semi Dry Transfer Cell (Bio-Rad). Nonspecific binding was blocked with 5% milk protein in TBS (20 mM tris, 136 mM NaCl, Ph 7.6) for 1 h before incubating the nitrocellulose membrane with one of the following antibodies: mouse monoclonal anti-claudin-1, - 4 and E-cadherin and rabbit polyclonal anti-claudin-3 and occludin (ZYMED, Laboratories Inc, USA) at 1:400 and goat anti claudin-6 and -9 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:500. All the dilutions were made in TTBS (0.1% Tween-20 in TBS). Horseradish peroxidase-labeled rabbit anti-goat Ig’s (1:10,000), goat anti-rabbit Ig’s (1:10,000) and goat anti-mouse Ig’s (1:4000) (all from Zymed) were used as secondary antibody in TTBS. Antibody binding was detected by chemilumi-niscence using ECL Western Blotting Detection System (Amersham Biosciences, Little Chalfont Bucks., UK). Since claudin-9 endogenous expression was not found in the cell membranes, it was excluded from the analysis.
The differences in claudin-1 expression in the cell membrane were determined with images obtained from the chemiluminiscence sheets using the MiniBIS Pro (DNR Bio-Imaging Systems, Jerusalem, Israel) and analyzed with the GelQuant software. The area under the curve for the basal and post-treatment results was determined by triplicate using the values obtained after measuring the amount of pixels/square inch, for each band.
Transepithelial electrical resistanceBriefly, cells were seeded onto Millipore Millicell cell culture inserts (0.4 um pore size) in 6 well plates until confluence. Huh-7 and Huh-7.5 monolayer formation in transwells was assessed after 48 h by measuring TER using the EVON multimeter (World Precision Instruments, Saratostra, FA) and chopstick-style electrodes. Growth medium was removed and the differentiating monolayers were gently washed twice with PBS before adding fresh culture medium containing PEG-IFN-α2b with or without ribavirin and left for various periods of time before measurements were made at room temperature. Replicates of three separate determinations were analyzed. The results are expressed by Ohm’s/cm2.
Semiquantitative RT-PCRTotal RNA was extracted from the cell cultures using Trizol reagent (Life Technologies Mexico, Mexico City), according to the manufacturer’s protocol. Concentration of RNA was determined at 260–280 nm using a NanoDrop 2000c Spectrophotometer (ThermoFisher Scientific, Waltham, MA). Samples (0.1 µg/µL RNA) were reverse-transcribed with the Invitrogen single step RT-PCR kit (Life Technologies). The RT-PCR cycle conditions were 30 min at 55 °C, 2 min at 94 °C, then 40 cycles at 94 °C for 20 s, 59.7 °C for 30 s, 72 °C for 40 s, and 7 min at 72 °C. Reaction products were resolved by electrophoresis in 1% agarose gels. Actin cDNA was used as internal standard to normalize RNA amounts. Primer sequences were:
- •
Actin forward 5’-TGAAGGTGACAGCAGTCGGTTG-3’.
- •
Actin-reverse 5’-GGCTTTTAGGATGGCAAGGG AC-3’.
- •
Claudin-1 forward 5’-TGCCCA CCTGCAAACTCTC-3’.
- •
Claudin-1 reverse 5’-GCCTCTGTGTCACACGTAGTC-3’.
Results were analyzed using Sigma-Stat. All values are expressed as the mean + standard deviation of the mean (SD). Student’s t-test analysis was used to evaluate differences between two groups. Differences were considered to be significant for values of p < 0.05.
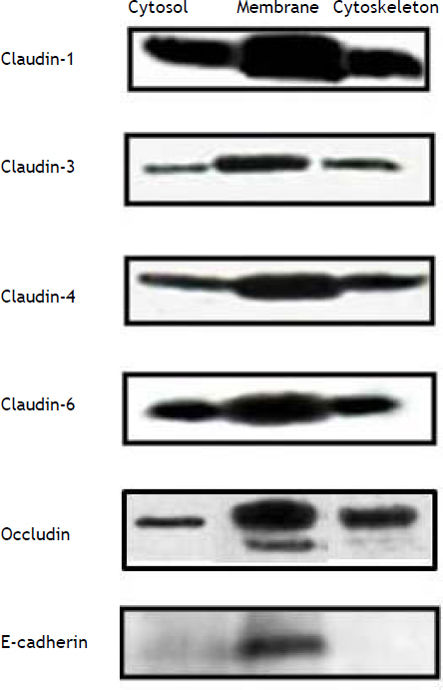
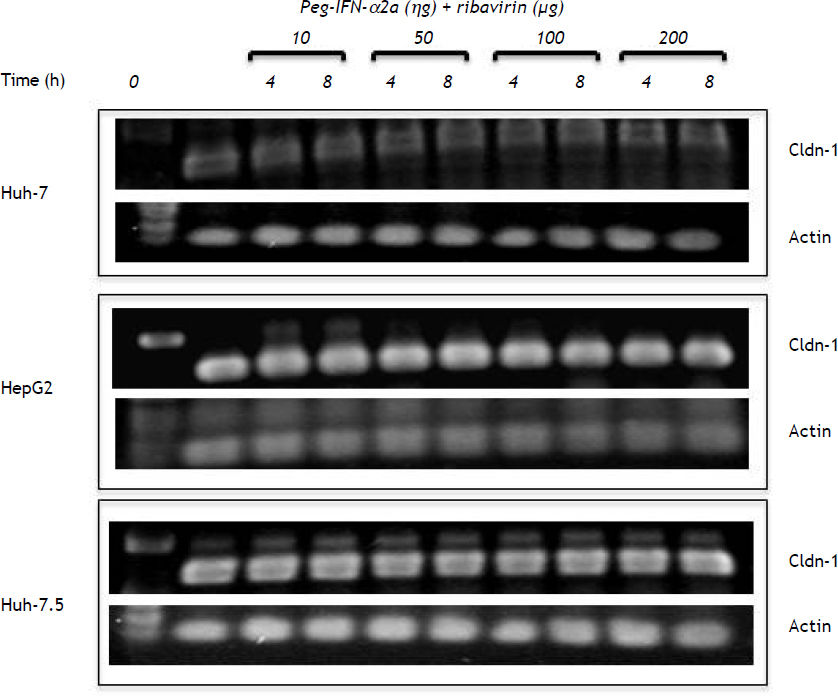
ResultsConstitutive expression of tight junction proteins in HepG2 cellsFigure 1 shows the cellular localization of claudins in HepG2 cells. Claudin-1, -3, -4, -6 and occludin were very strongly expressed in the cell membrane; the expression of these proteins was also observed in the cytosol and cytoskeleton fractions, but in a smaller amount in comparison with the cell membrane. E-cadherin was only expressed in the cell membrane. We evaluated these proteins because HCV utilizes cell membrane expressed tight junctions proteins to infect hepatocytes.7,8
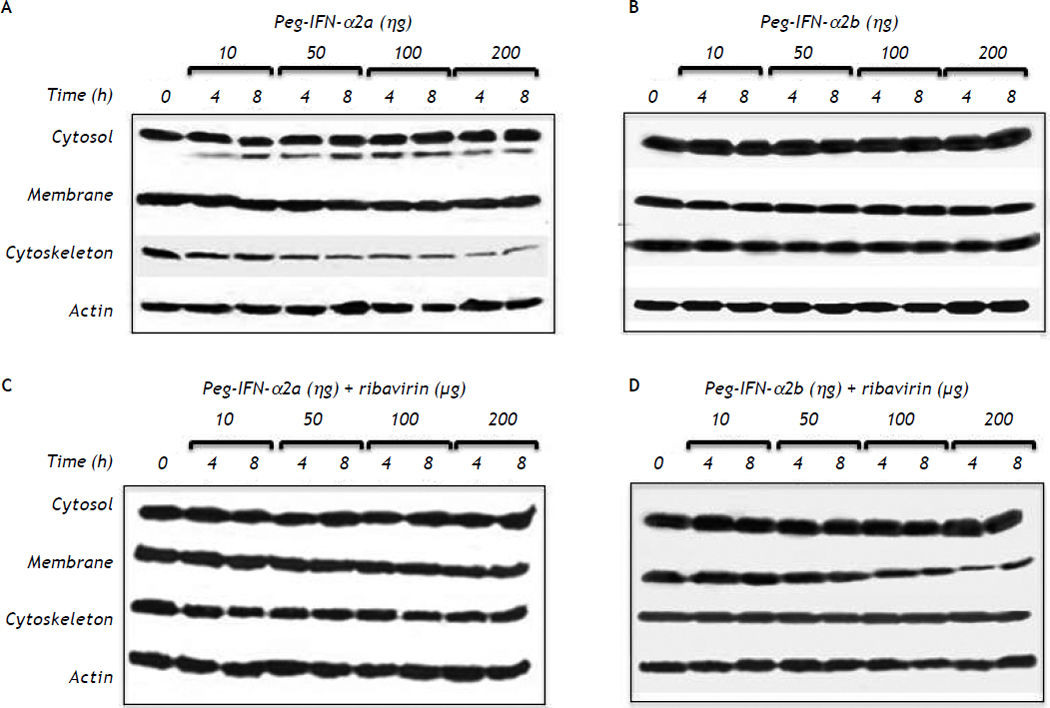
Effect of pegylated interferon and ribavirin on HepG2 cellsPEG-IFNα-2a did not modify the cell membrane expression of claudin-1 throughout the different experimental periods and treatments but induced a slow but progressive decrease in claudin-1 expression in the cytoskeleton fraction at concentrations as low as 10 ng/mL after 4 h of treatment (Figure 2A). The effect was clearly dose-dependent. The expression of claudin-1 in any of the cell fractions was not modified by PEG-IFNα2b at concentrations as high as 200 ng/mL (Figure 2B).
Effect of different type 1 pegylated interferons alone or in combination with ribavirin upon claudin-1 expression on HepG2 cells. A. Pegylated IFN-α2a. B. Pegylated IFN-α2b. C. Pegylated IFN-α2a + ribavirin. D. Pegylated IFN-α2b + ribavirin. The effect of the different doses evaluated was determined at two different exposure times. In C and D the dose of ribavirin was identical to the dose of the interferon being evaluated.
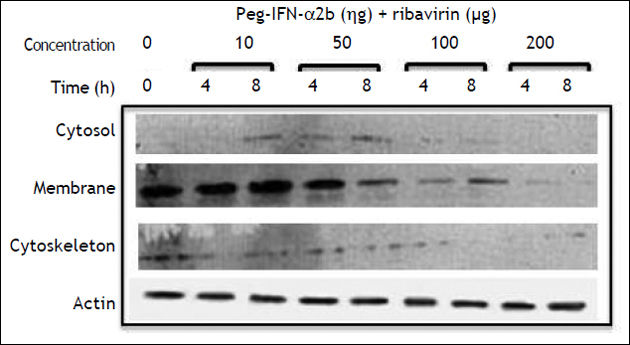
PEG-IFNα-2a + ribavirin treatment induced a trivial decrease in claudin-1 expression in the cell membrane fraction; the effect was initially observed at a 50 ng/mL concentration and an 8 h incubation period (Figure 2C). Interestingly, the cells treated with PEG-IFNα-2b + ribavirin showed a similar but more noticeable decrease in claudin-1 expression (Figure 2D) in the cell membrane fraction. The inhibitory effect was initially observed at a 50 ng/mL concentration and at 8 h incubation period in both treatment schemes and it was dose dependent as figure 2C clearly shows.
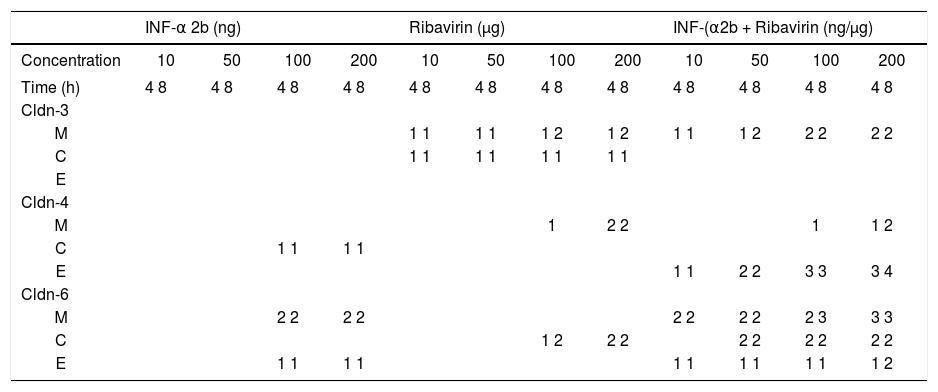
Table 1 summarizes the modifications observed in the expression of claudin-3, -4, and -6, in the cell fractions of HepG2 cells. PEG-IFNα-2b treated cells diminished claudin-4 expression in the cytosol fraction, and claudin-6 and -9 expression in the cell membrane fraction. Claudin-3 expression was not modified in any fraction. Ribavirin diminished the membrane expression of claudin-3 and -4 and the cytosol expression of claudin-3 and -6. The combined treatment with PEG-IFNα-2b + ribavirin diminished the membrane expression of claudin-3, but the effect was more pronounced in relation to claudin-6, since the treatment disturbed the overall expression of claudin-6 in the cytosol and the cytoskeleton. Occludin expression was not modified by pegylated interferon with or without ribavirin in none of the three cellular fractions analyzed (data not shown).
Effect of PEG-IFNα2b and/or ribavirin on the expression of other membrane expressed claudins in Hep G2 cells.
| INF-α 2b (ng) | Ribavirin (µg) | INF-(α2b + Ribavirin (ng/µg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 10 | 50 | 100 | 200 | 10 | 50 | 100 | 200 | 10 | 50 | 100 | 200 |
| Time (h) | 4 8 | 4 8 | 4 8 | 4 8 | 4 8 | 4 8 | 4 8 | 4 8 | 4 8 | 4 8 | 4 8 | 4 8 |
| Cldn-3 | ||||||||||||
| M | 1 1 | 1 1 | 1 2 | 1 2 | 1 1 | 1 2 | 2 2 | 2 2 | ||||
| C | 1 1 | 1 1 | 1 1 | 1 1 | ||||||||
| E | ||||||||||||
| Cldn-4 | ||||||||||||
| M | 1 | 2 2 | 1 | 1 2 | ||||||||
| C | 1 1 | 1 1 | ||||||||||
| E | 1 1 | 2 2 | 3 3 | 3 4 | ||||||||
| Cldn-6 | ||||||||||||
| M | 2 2 | 2 2 | 2 2 | 2 2 | 2 3 | 3 3 | ||||||
| C | 1 2 | 2 2 | 2 2 | 2 2 | 2 2 | |||||||
| E | 1 1 | 1 1 | 1 1 | 1 1 | 1 1 | 1 2 | ||||||
M: membrane fraction. C: cytosol fraction, and E: cytoskeleton fraction (see Material and methods). The number represents the relative decrease in claudin expression: 1, trivial; 2, weak; 3, strong; 4, very strong.
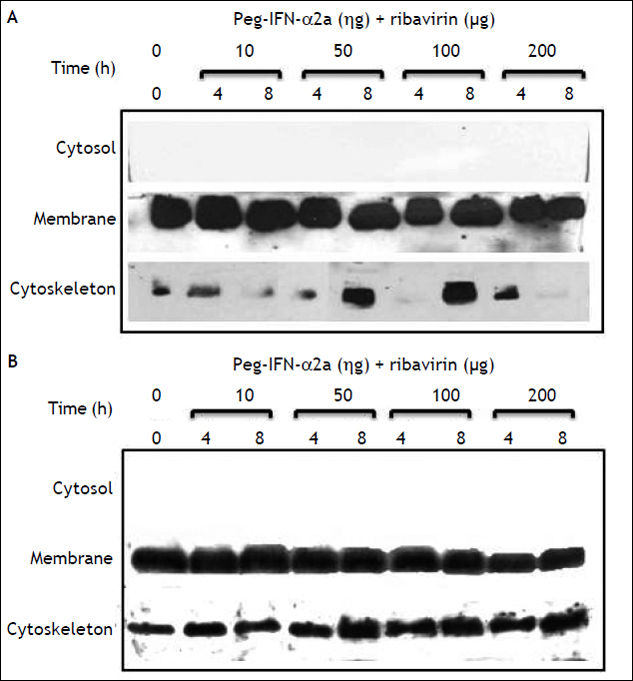
E-cadherin expression decreased importantly in the cell membrane fraction. The inhibitory effect started, similarly to claudin-1, at 50 ng/mL PEG-IFNα-2b + ribavirin concentration and after an 8 h incubation period. In contrast to claudin-1 expression, E-cadherin expression was completely abolished at 200 ng/mL of PEG-IFNα-2b + ribavirin (Figure 3). The inhibitory effect was only observed in the membrane cell fraction since the constitutive expression of this adhesion molecule in the cytosol and cytoskeleton fractions is negligible.
Effect of pegylated interferon and ribavirin on Huh-7 and Huh-7.5 cellsOn figures 4A and 4B, we show the constitutive expression of claudin-1 in the membrane and cytos-keleton fractions of Huh-7 and Huh-7.5 cells. Similarly to the inhibitory effect observed in HepG2 cells, the combined treatment with PEG-IFNα-2b + ribavirin diminished the membrane expression of claudin-1 but the decrease was only evident at 200 ng concentrations as opposed to 50 ng that was the inhibitory concentration in HepG2 cells. Densitometric analysis demonstrated a 20% decrease in claudin-1 cell membrane expression in Huh-7 PEG-IFNα-2b + ribavirin treated cells compared with Huh-7 cells without treatment, and around 50% for Huh-7.5 cells compared to controls. Interestingly, the decrease in claudin-1 cell membrane expression in the Huh-7.5 cells could probably be due at the expense of the cytoskeleton as the initial very weak expression observed in the first lane, that represents the constitutive expression, showed a progressive increase as the combined treatment doses were increased.
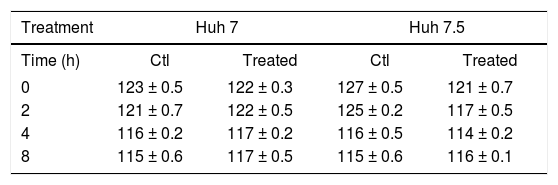
Effect of pegylated interferon and ribavirin on cells transepithelial electrical resistance (TER)Because claudin expression was modified by PEG-IFNα-2b + ribavirin treatment we measured TER before and after treatment. Table 2 shows that there was a very marginal diminution in TER values in the Huh-7 and Huh-7.5 cell lines in non-treated and PEG-IFNα-2b + ribavirin treated conditions. The differences were not statistically significant.
Effect of PEG-IFNα2b and ribavirin, on the TER values of Huh-7 and Huh-7.5 cell lines.
| Treatment | Huh 7 | Huh 7.5 | ||
|---|---|---|---|---|
| Time (h) | Ctl | Treated | Ctl | Treated |
| 0 | 123 ± 0.5 | 122 ± 0.3 | 127 ± 0.5 | 121 ± 0.7 |
| 2 | 121 ± 0.7 | 122 ± 0.5 | 125 ± 0.2 | 117 ± 0.5 |
| 4 | 116 ± 0.2 | 117 ± 0.2 | 116 ± 0.5 | 114 ± 0.2 |
| 8 | 115 ± 0.6 | 117 ± 0.5 | 115 ± 0.6 | 116 ± 0.1 |
The values are reported in ohms/cm2. The results represent the mean ± standard deviation of three separate determinations. The wells were treated with 200 ng of PEG-IFNα2b + 200 µg of ribavirin. None of the differences reached statistically significant differences.
To corroborate if the treatments modified mRNA levels, claudin-1 mRNA was analyzed. Figure 5 shows that the basal concentration of claudin-1 RNAm in Huh-7 cells was lower in comparison with HepG2 and Huh-7.5 cell lines. Interestingly, all the different PEG-IFNα-2b + ribavirin treatments did not modify claudin-1 mRNA expression in any of the three cell lines.
DiscussionHepatitis C virus (HCV) is a small enveloped positive stranded RNA virus that belongs to the Hepacivirus genus in the Flaviviridae family. More than 80% of acutely HCV-infected patients fail to eradicate the virus and subsequently develop chronic hepatitis,1,2 liver cirrhosis and hepatocellular carcinoma. HCV entry is dependent on the interaction between viral envelope glycoproteins, E1 and E2, and multiple host membrane molecules that include CD81, LDLr, SRCB-I, claudins, occludin and glycosamino-glycans.9,10,16 The first line of defense against HCV infection is provided by innate immune response cells, which secrete IFN-γ that inhibits replication of HCV.17,18 Endogenously produced type 1 interferons IFN-α and IFN-β are also effective inhibitors of HCV replication19 but their short circulating halflife (2–3 h in humans) makes frequent dosing necessary. Despite this, high-dose unmodified interferon-a therapy had a limited efficacy in naive patients20 partly because there was an increase in the frequency and severity of adverse effects so patient compliance was difficult to maintain; the combination of unmodified interferon and ribavirin in patients with chronic HCV infection who relapsed after treatment with interferon alone resulted in higher rates of SVR21 but the percentage of side effects was similar and the response was dose-dependent. Renal elimination is the predominant clearance mechanism of nonpegylated interferon but clearance after interacting with cellular interferon receptors has been shown.22 Pegylated interferon significantly decreases plasma drug clearance (35-77 h)23,24 providing higher concentrations that have led to reduce dosing frequency and improved responses. The first therapeutic scheme for HCV infection used non-pegylated interferon and the maximal sustained viral response (SVR) was in the 25% range. When type I pegylated interferons appeared SVR increased slightly, but when ribavirin was included as a therapeutic complement to pegylated type I interferons, the SVR increased to a 45-55% (25). The plasma concentration of ribavirin can be a predictor of efficacy and SVR to treatment26 but there is wide pharmacokinetic variability to pegylated type 1 interferon mainly driven by patient weight, so that the standard dose may not reach levels needed to achieve sustained viral response.27 In order to eliminate the influence of weight and renal clearance we evaluated pegylated interferon efficacy on a well-accepted cell model of HCV-infection.
HCV entry into the cell requires claudin-1, although to allow HCV entry into the hepatocyte, claudins need to interact with EWI-2wint, which blocks the E2-CD81 interaction.17 Occludin and claudins 6 and 9 also enable HCV entry into cells.8,9,16 Our results showed that pegylated interferon in combination with ribavirin diminished the cell membrane expression of claudin-1 in HepG2 cells. We also observed a decrease on claudins 3 and 4 membrane expression induced by ribavirin alone or in combination with PEG-IFN-α2b. Claudin-4 gene is important for interferon-inducible antiviral responses.28 Since claudin-1 mRNA was not affected by the combined treatment of PEG-IFN-α2b plus ribavirin, we assume that the decrease in claudin-1 and -4 expression could be related to alterations in the cytoskeleton, that possibly affect their transport to the cell membrane. Nevertheless, it has been shown that pro-inflammatory cytokines in highly inflammatory processes disrupt the distribution of claudin-1 in the cell membrane29 and this modification is crucial in the regulation of HCV cellular tropism.30 Claudin membrane disruption has also been observed with gamma interferon and tumor necrosis factor alpha, both of which induce internalization of claudin-1 and claudin-4.31
The membrane expression of E-cadherin, a protein associated with bacterial adhesion and cancer progression, was significantly reduced with PEG-IFNα-2b + ribavirin. Down regulation of the E-cad-herin gene has been associated with early hepatocellular carcinoma32 but the incidence of hepatocellular carcinoma in PEG-IFNα-2b/ribavirin treated patients is low. E-cadherin is a protein often adjacent to actin-containing filaments of the cytoskeleton; its inhibition alters the cadherin-based cell-cell junctions at sites where mechanical forces are sensed and elicit proportional cellular responses such as regulation of cell growth and cell fate,33 thus favoring the “isolation” and further elimination of the infected cells by immune response cells. Occludin expression, another tight junction protein utilized by the HCV to enter hepatocytes was not modified by ribavirin either alone or in combination with PEG-IFNα-2b. Occludin interacts with ZO-1 and ZO-2 proteins and is basically related to organization of the microtubule network, but since inflammatory mediators are emerging as regulators of the ability of hepatocytes to support HCV entry,10 our results are consistent with those of Mee, et al.,34 that have shown that IFN perturbs the integrity of the tight junction but has no discernable effect on HCV entry.
The transepithelial electrical resistance of PEG-IFNα-2b + ribavirin treated cells showed an inconspicuous decrease. HCV subgenomic replicon containing Huh cells do not represent the actual viral infection and replication process, and Huh7.5 cells do not synthetize the E2 protein that is vitally required by the HC viruses to infect new cells through the claudin-1 and CD81 pathway. The barrier function is not altered when epithelial cells are exposed to virally mutated envelope proteins.35 Interferon-gamma is known to regulate claudin-1 expression in Caco-2 cells infected with cell culture-derived HCV but it does not affect the TER values in this cell line.36,37
Since claudin-1 mRNA was not affected by the combined treatment we must assume that the diminished expression of claudin-1 at the membrane level of the cells must be secondary to an effect on the translation process induced by ribavirin, a guanosine nucleoside analogue that affects the mRNA of guanosyl transferase. Ribavirin affects translation in cells loaded with cyclin D1, GAPDH and VEGF mRNA by suppressing the translation initiation factor eIF4E.38 The HCV protein NS5A, hyper-phosphorylates eIF4E, without altering global translation.39
The apparently different inhibitory capacity of both pegylated interferons might be secondary to their distinct chemical structure.40,41 PEG-IFNα-2a has four domains that are responsible for the receptor binding interaction;42 the four major pegylation sites in PEG-IFN fall within these domains. Pegylation diminishes the affinity of the receptor-ligand interactions especially in PEG-IFNα-2a.43 Linear pegylated interferons such as PEG-IFNα-2b have a 93% higher (25-35 fold) specific in vitro antiviral activity than branched pegylated interferon such as PEG-IFNα-2a due to non-optimal interaction with IFN receptor.44,45 This might explain the differences between both pegylated interferons in relation to their ability to inhibit claudin-1 membrane expression, and thus explain differences in their antiviral activity. Nevertheless, this effect may not be universal since the inhibitory effect of interferon-a upon SRCB-1 expression46 is not modified by pegylation.
Our results add to the understanding of the possible antiviral mechanism of the combined pegylated interferon + ribavirin treatment used in chronic HCV infected patients. Nevertheless, the need for higher sustained viral responses in HCV infection has forced the search of new antiviral drugs since the use of pegylated interferons is being questioned.47
Financial SupportThis work was supported by grants IN-208910 (DGAPA, UNAM), S0008-2009-1-112628 and CB-2012-01-177678 (CONACYT, México), and Buffington’s de Mexico, S.A. de C.V.