Background. MUC2 and MUC5AC overproduction is considered to be associated with hepatolithiasis and related to inflammation. However, mechanisms underlying MUC upregulation under inflammatory stimulation in human intrahepatic biliary epithelial cells (HIBECs) are not completely understood.
Material and Methods. Expression of MUC2 and MUC5AC mRNA in HIBECs was detected by real-time PCR. Expression of COX-2, EP4, and phosphorylated ERK, JNK and p38MAPK protein was detected by Western blot. Concentrations of PGE2, IL-1ß and TNF-α in cell culture supernatants were measured using the Quantikine Elisa kit.
Results. COX-2 expression as well as PGE2 production in HIBECs was upregulated significantly by LPS, which was completely blocked by either TLR4 antagonist or NFkB inhibitor. Selective COX-2 inhibitor suppressed LPS-induced MUC2 and MUC5AC mRNA expression remarkably. Exogenous PGE2 increased MUC2 and MUC5AC mRNA expression in a dosage-dependent manner independent of IL-1ß and TNF-α. PGE2 receptor EP4 agonist elevated MUC2 and MUC5AC expression, whereas EP4 antagonist had the opposite effect. Expression of phosphorylated p38MAPK was upregulated by exogenous PGE2, and p38MAPK inhibitor reduced MUC2 and MUC5AC expression in HIBECs. In addition, it was found that levels of PGE2, MUC2 and MUC5AC in bile samples from the hepatic ducts affected by intrahepatic stones were significantly higher than those from the unaffected hepatic ducts of patients with hepatolithiasis.
Conclusions. Our findings indicate that PGE2 induces MUC2 and MUC5AC expression in HIBECs via EP4-p38MAPK signaling.
Mucins are a group of high molecular weight glycoproteins covering and protecting epithelial cells. Aberrant secretion of mucins or production of mucus core proteins (MUCs, backbone proteins of mucin) is usually associated with diseases of gastrointestinal tract, respiratory tract, and biliary tract. Among the identified MUCs, MUC2 and MUC5AC have been extensively studied. MUC2 protects intestinal epithelial cells from direct contact with bacteria, and MUC2 deficiency is involved in colitis.1–3 On the other hand, excessive production of MUC5AC is implicated in some pulmonary inflammatory diseases, such as asthma and chronic bronchitis.4–6
Roles of MUC2 and MUC5AC in biliary tract diseases have also been explored. They are both involved in cholecystitis, although MUC2 is not expressed in extrahepatic biliary tract in normal condition.7 In addition, MUC2 and MUC5AC overproduction seems to be associated with hepatolithiasis, because it was observed that human biliary epithelia with hepatolithiasis expressed more MUC2 and MUC5AC than those without hepatolithiasis.8 However, mechanisms underlying MUC upregulation by human intrahepatic biliary epithelial cells (HIBECs) are not completely understood.
It was observed that bile in patients with chronic proliferative cholangitis and hepatolithiasis contained high level of phospholipidase A2, PGE2 and MUCs.9 In addition, human biliary epithelia with hepatolithiasis were revealed to express an increased level of COX-2 and PGE2.10 Taken together, these studies suggest PGE2 may be involved in chronic proliferative cholangitis and subsequent hepatolithiasis. In the present study, we for the first time explored the role of PGE2 in MUC2 and MUC5AC overproduction by using cultured HIBECs.
Material and MethodsCell culture and cell viabilityHuman intrahepatic biliary epithelial cells (HI-BECs) were purchased from Sciencell (USA), and were cultured in medium supplied by the manufactor according to the manufactor’s protocol. Cell viability was more than 99% in in vitro experiments measured by trypan blue staining.
BileBile samples were obtained from the hepatic ducts affected by intrahepatic stones and the unaffected hepatic ducts of 15 patients with hepatolithiasis. Informed consent was obtained from all patients before collection of the samples which were stored at 80 °C until analysis. This study was carried out with the approval of the ethical committee of China Medical University.
Antibodies and reagentsAnti-COX-2, anti-EP4 and anti-b-actin were purchased from Santa Cruz. Anti-phospho-ERK, anti-phospho-JNK, and anti-phospho-p38MAPK were provided by R&D Systems. PGE2, IL-1ß and TNF-α Quantikine Elisa kits were obtained from R&D Systems. MUC5AC and MUC2 Quantikine Elisa kits were obtained from Boster (Wuhan, China). LPS, PGE2, NFkB inhibitor PDTC, specific COX-2 inhibitor NS-398, neutralizing TNF-α antibody inflixi-mab, were purchased from Sigma. selective EP1 agonist ONO-DI-004, selective EP2 agonist ONO-AE1-259, selective EP3 agonist ONO-AE-248, selective EP4 agonist ONO-AE1-329 and selective EP4 antagonist ONO-AE3-208 were provided by ONO Pharmaceutical. ERK inhibitor PD980595, JNK inhibitor SP600125 and p38MAPK inhibitor SB203580 were obtained from invivogen.
RNA extraction and reverse transcriptionTotal RNA was isolated from HIBECs by RNAiso Reagent (Takara) and reverse transcribed by PrimeScriptTM RT reagents Kit (Takara,) according to the manufacturer’s instructions, in a 10 μL reaction volume containing 5x PrimeScriptTM Buffer, 2 μL; PrimeScriptTM RT Enzyme Mix I, 0.5 μL; Oligo dT Primer (50 μM), 0.5 μL; Random 6 mers (100 μM), 0.5 μL; Total RNA, 500 ng and RNase Free dH2O.
Real-time PCRReal-time PCR was performed in 20 reaction mixtures containing SYBR Premix Ex TaqTM (Takara), 10 μL; PCR Forward Primer (10 μM), 0.4 μL; PCR Reverse Primer (10 μM), 0.4 μL; cDNA, 2 μL; dH2O, 7.2 μL, in an ABI 7500 Fast Real-Time PCR System (Applied Biosystems) according to the protocol supplied by the manufacturers. Primer sequences for MUC2 and MUC5AC were described in.11 Gene mRNA level was quantified by the comparative CT method, normalizing CT values of the target gene to those of housekeeping gene GAPDH.
Western blot20 μg protein was subjected to a 12% sodium dodecyl sulfate (SDS)/acrylamide gel, and transferred to a nitrocellulose membrane, which was blocked at 4 oC in PBS supplemented with 0.1% Tween and 5% milk powder. The membrane was then incubated with the primary antibody overnight. The ECL detection system was used to detect the signals. The band intensity was analyzed by Quantity One software.
ElisaConcentrations of PGE2, IL-1ß and TNF-a in cell culture supernatants (5 x 105) were detected by PGE2, IL-1ß and TNF-α Quantikine Elisa kits, respectively. Concentrations of PGE2, MUC5AC and MUC2 in bile were detected by PGE2, MUC5AC and MUC2 Quantikine Elisa kits, respectively. All ELISA detections were performed according to the manufacturer’s protocols. The detection limit is 6pg/ mL for PGE2, 1 pg/mL for IL-1ß and 4 pg/mL for TNF-α, MUC5AC and MUC2.
Statistical analysisDifferences in gene expression and protein contents in cell culture supernatant and bile were analyzed by Student’s t-test or one way ANOVA. Statistical analysis was carried out using SPSS v14.0 (SPSS, Chicago, IL). When P value was < 0.05, difference was considered as significant.
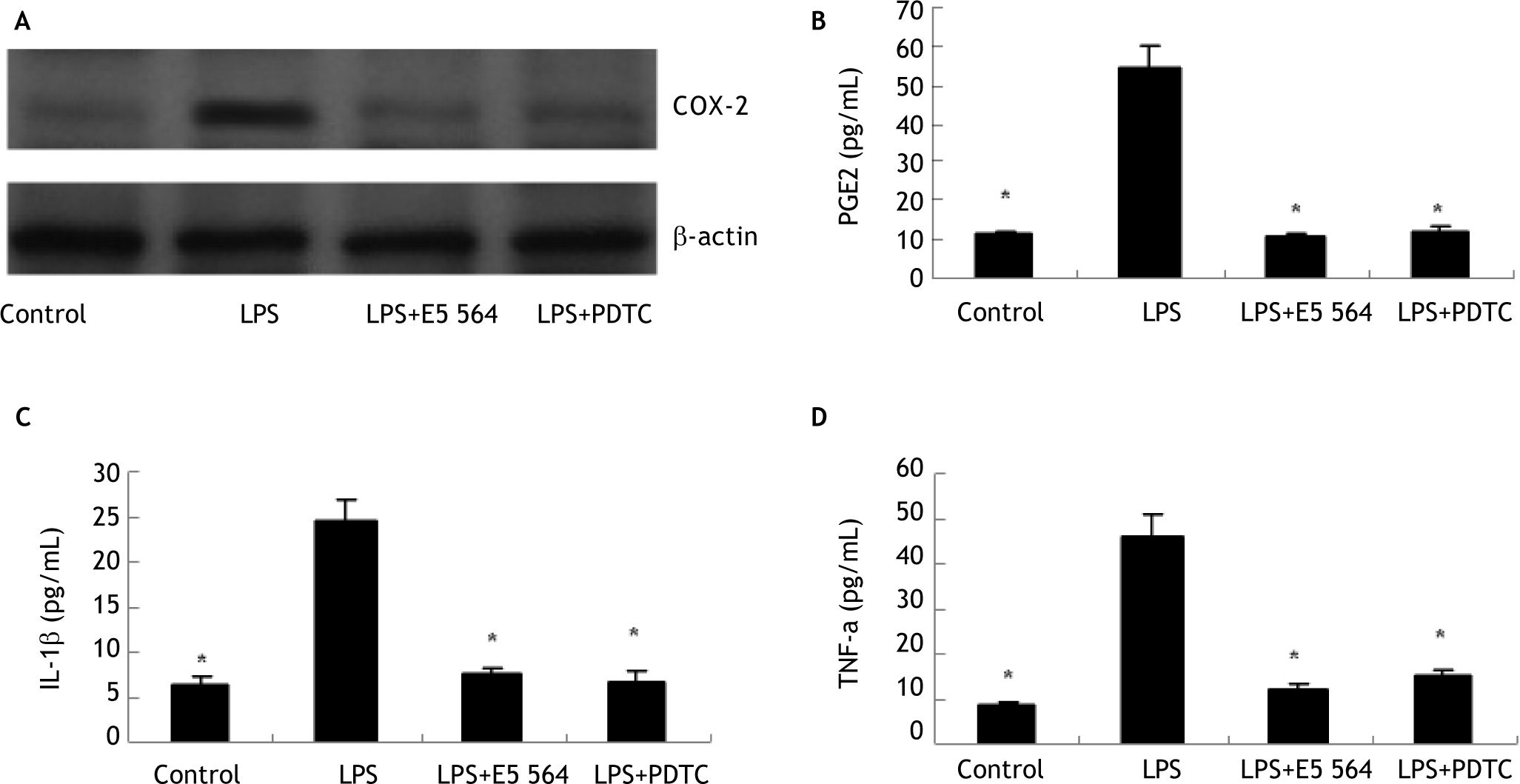
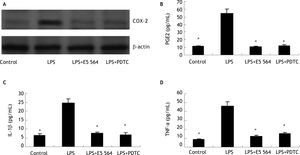
ResultsLPS stimulates COX-2 expression and PGE2 production in HIBECs in a TLR4-NFKB-dependent mannerTo investigate whether inflammatory stimuli induce HIBECs to express COX-2 and produce PGE2, we treated HIBECs with LPS (10 μg/mL) for 4 hours. Western blot detection revealed that COX-2 expression is upregulated significantly (Figure 1), and ELISA detection showed that HIBECs secreted more PGE2 into cell culture supernatant (Figure 1). However, the induced COX-2 expression as well as PGE2 production was completely blocked by either TLR4 antagonist E5564 (10 μmol/L) or NFkB inhibitor PDTC (100 μmol/L) (Figure 1), suggesting that TLR4-NFkB signaling was involved in the induction of COX-2/PGE2 by LPS.
Induction of COX-2 expression and PGE2 production in HIBECs by LPS in a TLR4-NFkB-dependent manner (A) LPS (10 μg/mL) induced COX-2 expression in HIBECs, which was blocked either by TLR4 antagonist E5564 (10 μmol/L) or NFkB inhibitor PDTC (100 μmol/L). B-D. LPS (10 μg/mL) induced PGE2, IL-1ßand TNF-α secretion in HIBECs, which was suppressed either by TLR4 antagonist E5564 (10 μmol/L) or NFkB inhibitor PDTC (100 μmol/L). *P < 0.01, vs. LPS. Data are expressed as mean ± SD, n = 3.
In addition, we also explored whether LPS could induce HIBECs to secret IL-1ß and TNF-a. ELISA detection indicated that LPS treatment (10 μg/mL) elevated levels of IL-1ß and TNF-α in culture supernatant of HIBECs (Figure 1). Similarly, the increased production of IL-1ß and TNF-a by HIBECs was also abrogated either by E5564 (10 μmol/L) or PDTC (100 μmol/L) (Figure 1), suggesting that induction of IL-1b and TNF-α by LPS in HIBECs was also dependent of TLR4-NFkB signaling.
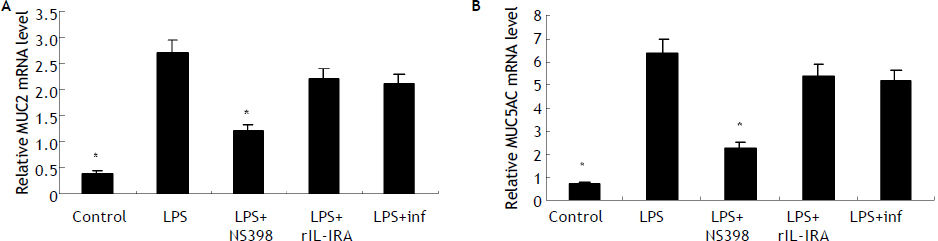
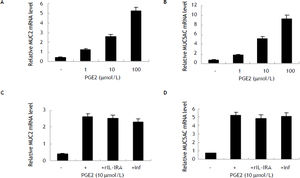
LPS induces MUC2 and MUC5AC expression mainly dependent endogenous PGE2 productionReal-time PCR detection indicated that HIBECs expressed more MUC2 and MUC5AC mRNA after treatment with LPS for 8 hours (10 μg/mL) (Figure 2). To exlore the role of PGE2 in the LPS-induced upregulation of MUC2 and MUC5AC, we pre-treated HIBECs with a selective COX-2 inhibitor, NS-398 (100 μmol/L). It was found that levels of MUC2 and MUC5AC mRNA in HIBECs decreased by 56% and 64%, respectively (Figure 2). Furthermore, we pre-treated HIBECs with neutralizing TNF-a antibody, infliximab (10 μg/mL) and recombinant IL-1 receptor antagonist (rIL-1RA, 5 μg/mL, R&D systems), respectively, to evaluate whether IL-1b and TNF-a were also involved in LPS-induced MUCs expression, because LPS also stimulated IL-1ß and TNF-α secretion in HIBECs. The results showed that MUC2 and MUC5AC expression decrease by 23% and 18%, respectively, by infliximab, and decreased by 18% and 15%, respectively by rIL-1RA (Figure 2).
Induction of MUC2 and MUC5AC expression in HIBECs by LPS mainly dependent endogenous PGE2 production (A) and (B) LPS (10 μg/mL) induced MUC2 and MUC5AC mRNA expression in HIBECs, which was remarkably suppressed by COX-2 inhibitor NS398 (100 μmol/L). *P < 0.01, vs. LPS. Data are expressed as mean ± SD, n = 3. inf: infliximab.
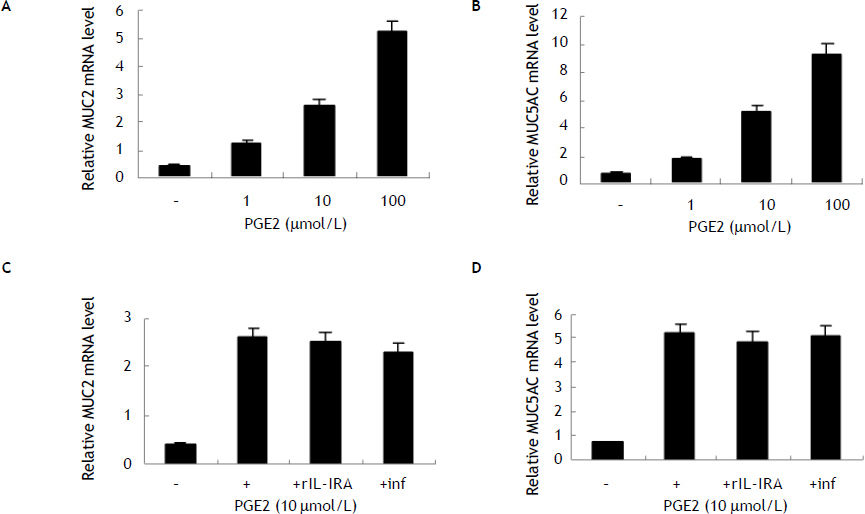
To further evaluate the role of PGE2 in MUC2 and MUC5AC expression, we treated HIBECs with exogenous PGE2 (1, 10 and 100 μmol/L, respectively). It was found that PGE2 increases MUC2 mRNA and MUC5AC mRNA expression in a dosage-dependent manner (Figure 3). In addition, neither infliximab (10 μg/mL) nor rIL-1RA (5 μg/mL) blocked exogenous PGE2-induced (10 μmol/L) MUC2 and MUC5AC expression, suggesting that PGE2 induced MUC2 and MUC5AC expression independent of IL-1ß and TNF-α (Figure 3).
Induction of MUC2 and MUC5AC expression in HIBECs by exogenous PGE2 (A) and (B) exogenous PGE2 (1, 10 and 100 μmol/L) stimulated MUC2 mRNA and MUC5AC mRNA expression in a dosage-dependent manner, P < 0.01, respectively. C and D. Neither rIL-1RA (5 μg/mL) nor infliximab (10 μg/mL) blocked exogenous PGE2-induced (10 μmol/L) MUC2 and MUC5AC expression. Data are expressed as mean ± SD, n = 3.
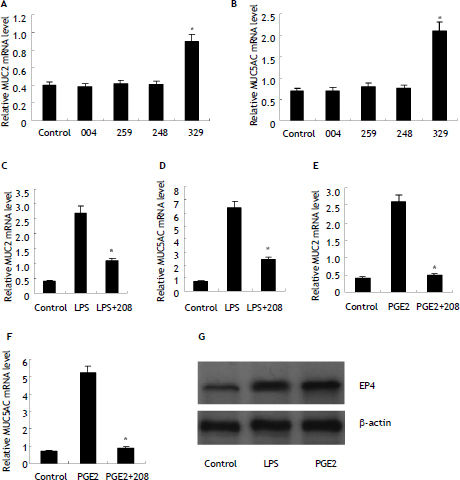
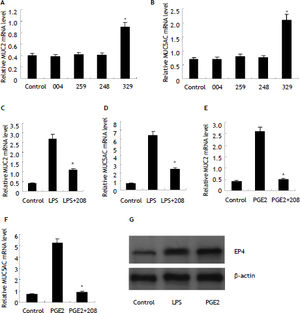
There are four receptors mediating extracellur PGE2 signals, EP1, EP2, EP3 and EP4. To investigate which receptor mediated PGE2 signals in HIBECs, we treated HIBECs with ONO-DI-004 (selective EP1 agonist, 5 μmol/L), ONO-AE1-259 (selective EP2 agonist, 5 μmol/L), ONO-AE-248 (selective EP3 agonist, 5 μmol/L) and ONO-AE1-329 (selective EP4 agonist, 5 μ-mol/L), respectively. It was found that only ONO-AE1-329 increased MUC2 and MUC5AC expression significantly (Figure 4). Furthermore, pre-treatment with EP4 antagonist ONO-AE3-208 (5 μmol/L) partially suppressed LPS-induced MUC2 and MUC5AC expression (Figure 4), and almost completely abrogated exogenous PGE2-induced MUC2 and MUC5AC upregulation (Figure 4). In addition, it was found that EP4 expression was enhanced either by LPS or exogenous PGE2 (10 μmol/L) (Figure 4).
POE2-induced MUC2 and MUC5AC upregulation mediated by EP4 receptor (A) and (B) selective EP4 agonist ONO-AE1-329 (5 μmol/L) stimulated MUC2 andMUC5AC mRNA expression in HIBECs. *P < 0.01, vs. control (C) and (D) EP4 antagonist ONO-AE3-208 (5 μmol/L) partially suppressed LPS-induced MUC2 and MUC5AC expression. *P < 0.01, vs. LPS. E and F. EP4 antagonist ONO-AE3-208 (5 μmol/L) abrogated exogenous POE2-induced MUC2 and MUC5AC expression. *P < 0.01, vs. PGE2. G. LPS (10 μg/mL) and exogenous PGE2 (10 μmol/L) enhanced EP4 expression in HIBECs. Data are expressed as mean ± SD, n = 3. 004: ONO-DI-004; 259: ONO-AE1-259; 248: ONO-AE-248; 329: ONO-AE1-329; 208: ONO-AE3-208.
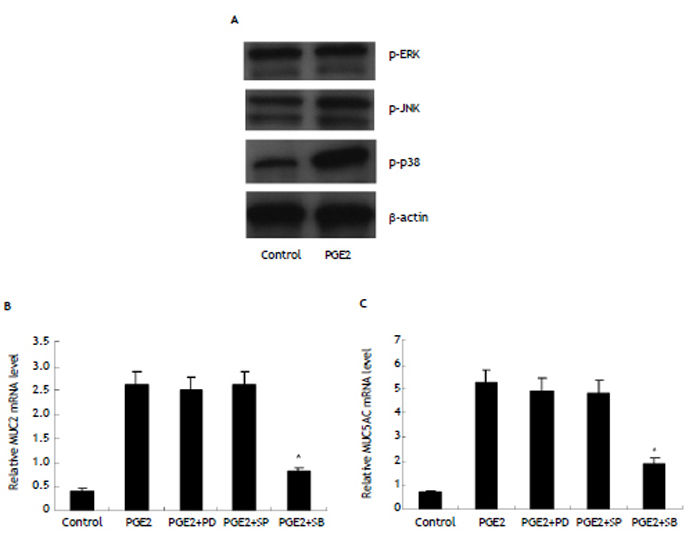
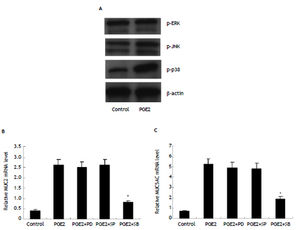
To explore the intracellular signal linking PGE2/ EP4 with MUC2 and MUC5AC, we examined expression of phosphorylated ERK, phosphorylated JNK and phosphorylated p38MAPK in HIBECs treated with exogenous PGE2 (10 μmol/L). It was found that only phosphorylated p38MAPK expression was remarkably increased (Figure 5). Furthermore, we treated HI-BECs with ERK inhibitor PD980595 (100 μmol/L, invi-vogen), JNK inhibitor SP600125 (50 μmol/L, invivogen), and p38MAPK inhibitor SB203580 (10 μmol/L, invivogen), respectively. We found that SB203580 significantly inhibited MUC2 and MUC5AC expression induced by exogenous PGE2, whereas PD98059 and SP600125 did not (Figure 5).
MUC2 and MUC5AC upregulation by PGE2/EP4 in HIBECs requiring P38MAPK activation (A) expression of phosphorylated p38MAPK in HIBECs was enhanced by exogenous PGE2 (10 μmol/L). B and C. P38MAPK inhibitor SB203580 (10 μmol/L) significantly suppressed exogenous PGE2- induced MUC2 and MUC5AC expression. *P < 0.01, vs. PGE2. Data are expressed as mean ± SD, n = 3. p-ERK: phosphorylated ERK. p-JNK: phosphorylated JNK. p-p38: phosphorylated p38MAPK. PD: PD980595; SP: SP600125. SB: SB203580.
In addition, we also detected concentrations of PGE2, MUC2 and MUC5AC in bile samples. ELISA detection revealed that levels of PGE2, MUC2 and MUC5AC were 268.8 ± 40.5 pg/mL, 48.2 ± 8.2 pg/mL and 69.6 ± 11.8 pg/mL in bile from the hepatic ducts affected by intrahepatic stones, which were significantly higher than those in bile from the unaffected hepatic ducts, 84.9 ± 10.4 pg/mL, 16.2 ± 4.3 pg/mL and 18.6 ± 5.7 pg/mL (P < 0.01, respectively).
DiscussionAs an intractable disease with frequent recurrence after operative interventions, hepatolithiasis is prevalent in East Asia including China. To date, the pathogenesis of this disease is still not completely understood. Studies have shown that hepatolithiasis is usually associated with chronic proliferative cholangitis,12–14 in which the biliary epithelia are hyperplastic and secret excessive mucin into biliary lumen. In fact, overproduction of MUCs is thought to play an important role in hepatolithiasis. It was found that bile in patients with hepatolithiasis contained high level of MUCs.9 In addition, biliary epi-thelia with hepatolithiasis were observed to express more MUCs than those without hepatolithiasis, especially, MUC2 and MUC5AC.8
However, mechanisms underlying MUC overproduction in epithelia remain largely unknown. Increasing evidence suggests that inflammatory stimuli may be involved in this process. For instance, IL-1ß and TNF-α were found to stimulate MUC5AC expression in human nasal epithelial cells via ERK/p38MAPK pathways.15 In addition, IL-1ß was also found to induce MUC5AC production by human tracheobronchial epithelial cells through cAMP-PKA signaling.16 These results indicate excessive production of MUC5AC is closely associated with pulmonary inflammatory diseases. MAPK-de-pendent MUC5AC upregulation was also observed in otitis media. Lee, et al. reported that Streptococcus pneumoniae induced middle ear epithelial cells to overproduce MUC5AC in vitro and in vivo.17
Studies have shown that inflammation is also involved in MUC expression in biliary epithelial cells. Vilkin, et al. reported that gallbladder epithelia with inflammation expressed a higher level of MUC5AC, which was associated with pigmented stones.7 Mechanisms underlying MUC5AC expression by gallbladder epithelia may be related to TNF-α and epidermal growth factor pathway.18 In addition, inflammatory stimuli were also found to induce intrahepatic biliary epithelia to express MUCs. Zen, et al. showed that LPS could induce overexpression of MUC2 and MUC5AC in murine biliary epithelial cells via TNF-α and protein kinase C.11 Furthermore, bacterial components and TNF-a were found to play important roles in MUC2 expression via CDX2 upregulation in cultured murine biliary epithelial cells.19,20
In the present study, we explored the roles of inflammation in MUC production and hepatolithiasis in HIBECs. Our study showed that LPS-induced MUC2 and MUC5AC overproduction was mainly mediated by inflammatory mediator PGE2, although other cytokines, such as IL-1ß and TNF-α, also seemed to be involved in this process, on the basis of the finding that COX-2/PGE2 inhibitor reduced MUC2 and MUC5AC expression by 56% and 64%, respectively, far higher than 23% and 18% by TNF-α inhibitor, and 18% and 15% by IL-1ß blocker. In addition, neither IL-1ß blocker nor TNF-α inhibitor suppressed exogenous PGE2-induced MUC overexpression, indicating that PGE2 exerts its positive effect on MUC expression independent of these two inflammatory cytokines.
Four G-protein coupled receptors, EP1, EP2, EP3 and EP4, have been found to mediate extracellular PGE2 signals in physiological and pathological conditions.21,22 In this study, we found that only EP4 agonist increased MUC2 and MUC5AC expression significantly. Moreover, pre-treatment with EP4 antagonist suppressed MUC2 and MUC5AC expression induced by either endogenous or exogenous PGE2. These results showed that EP4 mediated MUC overexpression in HIBECs by PGE2. Interestingly, it was found that PGE2 could upregulate EP4 expression in HIBECs, and then enhance the effect of PGE2 on MUC expression. Intracellular signal linking PGE2/EP4 with MUC2 and MUC5AC expression was also explored in this study. P38MAPK was identified as a candidate, because expression of phosphorylated p38MAPK was upregulated remarkably by exogenous PGE2, whereas expression of phosphorylated ERK and phosphorylated JNK was not, and among inhibitors of p38MAPK, ERK and JNK, only p38MAPK inhibitor significantly inhibited MUC2 and MUC5AC expression induced by exogenous PGE2.
In summary, we for the first time used cultured human biliary epithelial cells to explore the molecular mechanisms underlying MUC overproduction in inflammatory conditions. Our study suggests that PGE2 may play a crucial role in inflammation-associated human hepatolithiasis via EP4-p38MAPK signaling. At the same time, this study provides some putative therapeutic targets for preventing MUC overproduction. As shown in the study, MUC2 and MUC5AC overexpression can be blocked efficaciously by COX-2 inhibitor, EP4 antagonist and p38MAPK inhibitor.