Many anti-diabetic herbal preparations have been recommended in alternative systems of medicine for the treatment of diabetes. No systematic study has been done on the anti-diabetic efficacy of Byesukar, a polyherbal formulation to treat diabetes. The anti-diabetic efficacy of byesukar ethanol extract was evaluated in an animal model of diabetes induced by alloxan. Male Wistar rats were divided in to four groups. Group 1 was normal control group; group 2 and 3 received alloxan. After inducing experimental diabetes group 2 served as diabetic control; group 3 received byesukar (500 mg/kg body weight) orally for 30 consecutive days. Group 4 were normal rats which received byesukar extract alone. The effect of byesukar on glucose level in diabetic rats was studied and the level of glucose metabolizing enzymes (Hexokinase, glucose-6-phosphatase and fructose 1, 6-bisphosphatase) in the liver and kidney were estimated. The effect of byesukar on the serum and tissue lipid profile (Cholesterol, triglycerides, phospholipids and free fatty acids) were also estimated in diabetic rats. Our results indicate that treatment with byesukar resulted in significant reduction of blood glucose, tissue glucose-6-phosphatase and fructose 1, 6-bisphosphatase activity. The decreased tissue hexokinase activity in diabetes state was found to be significantly increased by byesukar treatment. Also the byesukar treated diabetic rats showed a significant decrease in the tissue lipid profile compared to the diabetic rats. In conclusion the decreased blood glucose accompanied with decreased lipid profile and changes in the activities of the glucose metabolizing enzymes shows the antidiabetic effect of byesukar.
Diabetes mellitus is a syndrome, initially characterized by a loss of glucose homeostasis resulting from defects in insulin secretion, insulin action both resulting impaired metabolism of glucose and other energy yielding fuels such as lipids and protein.1 Liver is an insulin dependent tissue, which plays a pivotal role in glucose and lipid homeostasis and is severely affected during diabetes.2 Liver participates in the uptake, oxidation and metabolic conversion of free fatty acids, synthesis of cholesterol, phospholipids and triglycerides. During diabetes a profound alteration in the concentration and composition of lipid occur.3 Decreased glycolysis, impeded glycogenesis and increased gluconeogenesis are some of the changes of glucose metabolism in the diabetic liver.4 Despite the great strides that have been made in the understanding and management of diabetes, the disease and disease related complications are increasing unabated.5 Death rates are twice as high among middle-aged persons (i.e., persons aged 45-60 years) with diabetes than among those without diabetes. Mortality from diabetes is related primarily to heart disease. Other complications also lead to increased morbidity and mortality rates. Risk for stroke is 2-4 times higher among persons with diabetes. Diabetes is the leading cause of new cases of blindness among adults aged 20-74 years and the leading cause of end-stage renal disease. Inspite of the presence of known antidiabetic medicine in the pharmaceutical market, remedies from medicinal plants are used with success to treat this disease.6Many traditional plant remedies for diabetes are used throughout the world. Plant drugs7 and herbal formulation8-10 are frequently considered being less toxic and more free from side effects than synthetic one. Few of the traditional plant treatments for diabetes have received scientific scrutiny and the World Health Organization (WHO) has recommended that this area warrants attention.11
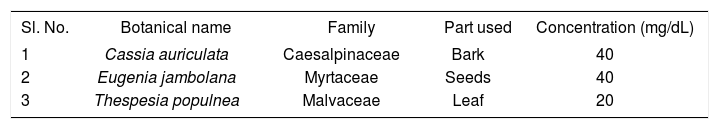
In the traditional system of Indian medicine, plant formulation and combined extracts of plants are used as drug of choice rather than individual. Various herbal formulations such as diamed,12 coagent db,13 hyponidd14 and diasulin,15 are well known for their antidiabetic effects. Byesukar is a polyherbal drug composed of three medicinal plants (Table I), which are already reported for their anti-diabetic activity. In our previous study, we have demonstrated the anti-diabetic effect of byesukar in alloxan diabetic rats.16 The present investigation was undertaken to study the effect of the potential antidiabetic herbal formulation, byesukar on blood glucose, tissue lipids and glucose metabolism in alloxan-diabetic rats.
Experimental proceduresAnimalsTwenty four male Wistar rats of body weights ranging from 150-200g were obtained from PSG Institute of Medical Sciences, Coimbatore. The animals were fed with standard pellet diet (Sai Durga Feeds, Bangalore, India) and water ad libitum. They were maintained in a controlled environment (12:12 h light/dark cycle) and temperature (30 ± 2 °C). The experimental protocol was approved by the Institutional Animal Ethical Committee, Government of India.
Drug and chemicalsByesukar, a polyherbal formulation was a gift from A.Z. Siddha Medicines, Chennai, India. 250 g of herbal drug powder were soaked overnight in 750 ml of 95% ethanol. The suspension was filtered and the residue was resuspended in an equal volume of 95% ethanol for 48 h and filtered again. The two filtrates were pooled and the solvents were evaporated in a rotary evaporator at 40-50 °C under reduced pressure. The yield of the extract was 12%. The extract was suspended in 0.1% gum acacia and used for this study.
Alloxan monohydrate was purchased from BDH Chemicals, Poole, England. All other biochemicals and chemicals used for the experiments were of analytical grade.
Experimental induction of diabetes in ratsThe rats (n = 18) were injected with alloxan monohydrate dissolved in sterile normal saline at a dose of 120 mg/kg body weight. After two weeks, rats with moderate diabetes having glycosuria (indicated by Benedict’s qualitative test) and hyperglycemia (i.e. with a blood glucose of 200-300 mg/dL) were used for the experiment.
Experimental designThe rats (n = 24) were divided into four groups of six animals each
Group 1 = Normal rats administered with gum acacia (n = 6)
Group 2 = Diabetic control rats (n = 6) Group 3 = Diabetic rats administered with byesukar (500 mg/kg body weight) daily for 30 days (oral) (n = 6)
Group 4 = Normal rats administered with byesukar (500 mg/kg body weight) daily for 30 days (oral) (n = 6)
At the end of 30 days, all the rats were euthanized under mild chloroform anaesthesia. Blood was collected and used for the estimation of blood glucose. Liver and kidney were immediately dissected out, washed in ice-cold saline to remove the blood. Lipids were extracted from serum and tissues by the method of Folch et al17 using chloroform-methanol mixture (CHCl3: MeOH) (2:1 v/v).
Total cholesterol was estimated by the method of Zlatkis et al18 and triglycerides by the method of Foster and Dunn.19 Free fatty acids and phospholipids were analyzed by the method of Falholt et al20 and Zilversmit et al.21
Hexokinase, glucose-6-phosphatase and fructose-1, 6-bisphosphatase were assayed by the method of Brandstrup et al,22 Koida and Oda23 and Gancedo et al24 respectively.
Statistical analysisThe data for various biochemical parameters were analyzed using analysis of variance (ANOVA) and the group means were compared by Duncan’s Multiple Range Test (DMRT). Values with p < 0.05 were considered statistically significant.
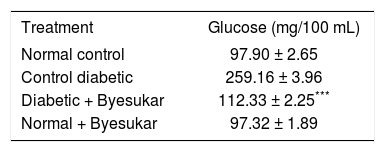
ResultsResults in table II shows the levels of blood glucose of normal and experimental rats. There was a significant elevation in blood glucose in alloxan diabetic rats compared to normal rats. Administration of byesukar significantly (p < 0.01) decreased the blood glucose level towards the normal.
Effect of Byesukar on the blood glucose of control and experimental rats.
| Treatment | Glucose (mg/100 mL) |
|---|---|
| Normal control | 97.90 ± 2.65 |
| Control diabetic | 259.16 ± 3.96 |
| Diabetic + Byesukar | 112.33 ± 2.25*** |
| Normal + Byesukar | 97.32 ± 1.89 |
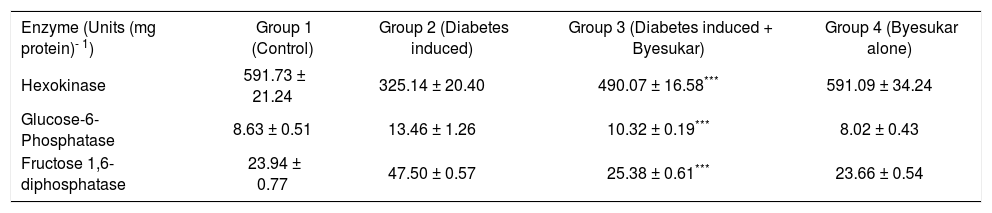
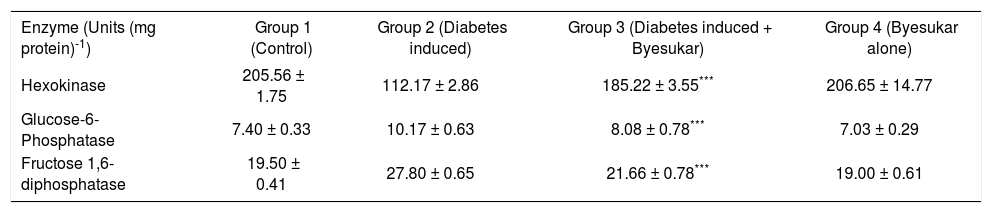
The activities of carbohydrate enzymes in liver and kidney are represented in tables IIIandIV respectively. Activity of hexokinase in the tissue decreased markedly in diabetic control rats. Treatment with byesukar significantly (p < 0.001) increased the hexokinase activity. The activity of gluconeogenic enzymes, glucose-6-phosphatase and fructose-1,6-bisphosphatase showed a marked increase in the tissue of diabetic control rats. Treatment with byesukar significantly (p < 0.001) decreased the glucose-6-phosphatase and fructose-1,6-bisphosphatase activity.
Effect of byesukar animals. on hexokinase, glucose-6-phosphatase and fructose-1, 6-bisphosphatase in liver of control and experimental animals.
| Enzyme (Units (mg protein)- 1) | Group 1 (Control) | Group 2 (Diabetes induced) | Group 3 (Diabetes induced + Byesukar) | Group 4 (Byesukar alone) |
|---|---|---|---|---|
| Hexokinase | 591.73 ± 21.24 | 325.14 ± 20.40 | 490.07 ± 16.58*** | 591.09 ± 34.24 |
| Glucose-6-Phosphatase | 8.63 ± 0.51 | 13.46 ± 1.26 | 10.32 ± 0.19*** | 8.02 ± 0.43 |
| Fructose 1,6-diphosphatase | 23.94 ± 0.77 | 47.50 ± 0.57 | 25.38 ± 0.61*** | 23.66 ± 0.54 |
Effect of Byesukar animals. on hexokinase, glucose-6-phosphatase and fructose-1, 6-bisphosphatase in the kidney of control and experimental animals.
| Enzyme (Units (mg protein)-1) | Group 1 (Control) | Group 2 (Diabetes induced) | Group 3 (Diabetes induced + Byesukar) | Group 4 (Byesukar alone) |
|---|---|---|---|---|
| Hexokinase | 205.56 ± 1.75 | 112.17 ± 2.86 | 185.22 ± 3.55*** | 206.65 ± 14.77 |
| Glucose-6-Phosphatase | 7.40 ± 0.33 | 10.17 ± 0.63 | 8.08 ± 0.78*** | 7.03 ± 0.29 |
| Fructose 1,6-diphosphatase | 19.50 ± 0.41 | 27.80 ± 0.65 | 21.66 ± 0.78*** | 19.00 ± 0.61 |
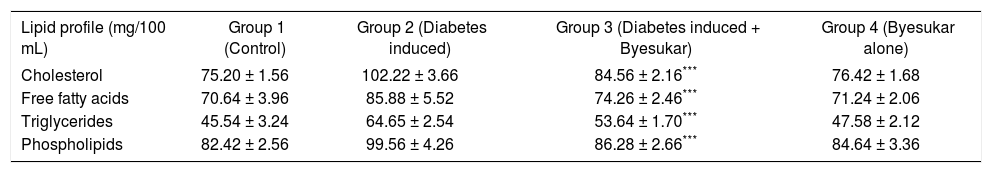
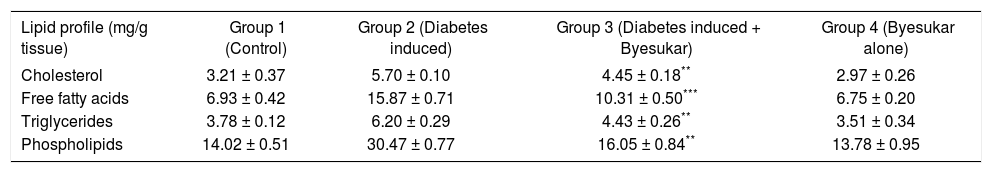
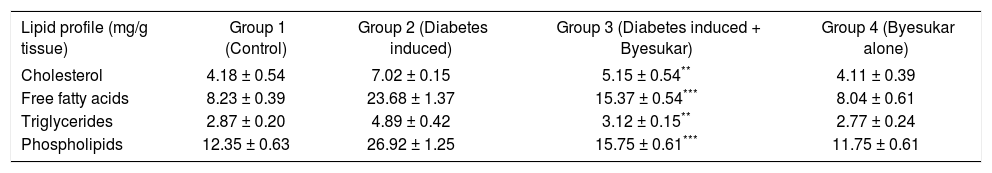
Tables V, VIandVII shows the levels of cholesterol, free fatty acids, triglycerides and phospholipids in serum, liver and kidney of control and experimental rats respectively. Serum, liver and kidney of diabetic rats showed significantly increased levels of cholesterol, free fatty acids, triglycerides and phospholipids, when compared to normal rats. In rats treated with byesukar, there was a significant (P < 0.001) decrease in the content of cholesterol, free fatty acids, triglycerides and phospholipids in the serum as well as tissues, when compared with diabetic control rats.
Effect of Byesukar on the levels of cholesterol, free fatty acids, triglycerides and phospholipids in the serum of control and experimental animals.
| Lipid profile (mg/100 mL) | Group 1 (Control) | Group 2 (Diabetes induced) | Group 3 (Diabetes induced + Byesukar) | Group 4 (Byesukar alone) |
|---|---|---|---|---|
| Cholesterol | 75.20 ± 1.56 | 102.22 ± 3.66 | 84.56 ± 2.16*** | 76.42 ± 1.68 |
| Free fatty acids | 70.64 ± 3.96 | 85.88 ± 5.52 | 74.26 ± 2.46*** | 71.24 ± 2.06 |
| Triglycerides | 45.54 ± 3.24 | 64.65 ± 2.54 | 53.64 ± 1.70*** | 47.58 ± 2.12 |
| Phospholipids | 82.42 ± 2.56 | 99.56 ± 4.26 | 86.28 ± 2.66*** | 84.64 ± 3.36 |
Effect of Byesukar on the levels of cholesterol, free fatty acids, triglycerides and phospholipids in the liver of control and experimental animals.
| Lipid profile (mg/g tissue) | Group 1 (Control) | Group 2 (Diabetes induced) | Group 3 (Diabetes induced + Byesukar) | Group 4 (Byesukar alone) |
|---|---|---|---|---|
| Cholesterol | 3.21 ± 0.37 | 5.70 ± 0.10 | 4.45 ± 0.18** | 2.97 ± 0.26 |
| Free fatty acids | 6.93 ± 0.42 | 15.87 ± 0.71 | 10.31 ± 0.50*** | 6.75 ± 0.20 |
| Triglycerides | 3.78 ± 0.12 | 6.20 ± 0.29 | 4.43 ± 0.26** | 3.51 ± 0.34 |
| Phospholipids | 14.02 ± 0.51 | 30.47 ± 0.77 | 16.05 ± 0.84** | 13.78 ± 0.95 |
Effect of Byesukar on the levels of cholesterol, free fatty acids, triglycerides and phospholipids in the kidney of control and experimental animals.
| Lipid profile (mg/g tissue) | Group 1 (Control) | Group 2 (Diabetes induced) | Group 3 (Diabetes induced + Byesukar) | Group 4 (Byesukar alone) |
|---|---|---|---|---|
| Cholesterol | 4.18 ± 0.54 | 7.02 ± 0.15 | 5.15 ± 0.54** | 4.11 ± 0.39 |
| Free fatty acids | 8.23 ± 0.39 | 23.68 ± 1.37 | 15.37 ± 0.54*** | 8.04 ± 0.61 |
| Triglycerides | 2.87 ± 0.20 | 4.89 ± 0.42 | 3.12 ± 0.15** | 2.77 ± 0.24 |
| Phospholipids | 12.35 ± 0.63 | 26.92 ± 1.25 | 15.75 ± 0.61*** | 11.75 ± 0.61 |
The diabetogenic effects of alloxan are attributed to a specific cytotoxic action mediated by hydroxyl radical generation on pancreatic β-cells. This damages a large number of β-cells resulting in a decrease in endogenous insulin release. Alloxan-administered rats therefore become hyperglycaemic in a short period of time, followed by hepatic glucose over production.25 In our study, we have found that byesukar treatment decreased the blood glucose in alloxan-diabetic rats. The possible mechanism of action of byesukar could be correlated with the reminiscent effect of the hypoglycemic sulphonylureas that promote insulin secretion by closure of K+-ATP channels, membrane depolarization and stimulation of Ca2+ influx, an initial key step in insulin secretion. In this context, number of other plants has also been reported to have antihyperglycemic and insulin stimulatory effects.26,27
The liver is regarded as one of the central metabolic organs in the body, regulating and maintaining homeostasis. It performs most of the reactions involved in the synthesis and utilization of glucose. The balance between glucose production and its utilization in the liver is regulated primarily by insulin. Liver is an insulin-dependent tissue and is severely affected during diabetes. In experimental diabetes, enzymes of glucose metabolism are markedly altered. Persistent hyperglycemia is a major contribution to such metabolic alterations that lead to the pathogenesis of diabetic complications.28
Diabetes results in a decrease in glucose utilization and an increase in glucose production in insulin-dependent tissues such as liver.29 Decreased glycolysis, impeded glycogenesis and increased gluconeogenesis are some of the changes of glucose metabolism in the diabetic liver.30 Hexokinase is an insulin-dependent and insulin-sensitive enzyme and are almost completely inhibited or inactivated in diabetic rat liver in the absence of insulin.28Decreased enzymatic activity of hexokinase and phosphofructokinase has also been reported in diabetic animals, resulting in depletion of liver and muscle glycogen.31,32 In our study, we also have observed decrease in hepatic as well as renal hexokinase activity in alloxandiabetic rats. Administration of byesukar to alloxan treated rats resulted in an increased activity of hexokinase in liver and kidney. This increased activity of hexokinase can cause the increased utilization of glucose for energy production. Byesukar has been observed to decrease the level of blood glucose. The decrease in the concentration of glucose in alloxan-treated rats given byesukar may be as a result of increased glycolysis (increased liver hexokinase activity).
Two gluconeogenic enzymes, glucose-6-phosphatase and fructose-1,6-bisphosphatase have been measured in the liver and kidney of diabetic animals and those treated with byesukar. Both enzymes showed an increase in activity during diabetes in the liver. Administration of byesukar was found to be more effective in reversing both the enzymes to normal levels in the liver of alloxan-diabetic rats. The increased hepatic as well as renal fructose1,6-bisphosphatase activity may be due to the changes in the allosteric effectors of the enzymes namely fructose-2,6-bisphosphate, ATP, AMP and citrate. In a diabetic state, there is more lipolysis than lipogenesis, especially in liver, which will result in the formation of more AMP and lower utilization of citrate for lipogenesis leading to high energy state in the cell, i.e. higher concentration of ATP is more favorable for fructose-1,6-bisphosphatase activation.30 The reduction in the activities of these gluconeogenic enzymes can result in decreased concentration of blood glucose. Administration of byesukar had increased the activity of hexokinase and decreased the activities of both glucose-6-phosphatase and fructose-1, 6-bisphosphatase in alloxan diabetic rats.
Excess of fatty acids in serum produced during diabetes promotes conversion of excess fatty acids into phospholipids and cholesterol in liver. These two substances along with excess triglycerides formed at the same time in liver may be discharged into blood in the form of lipoproteins.29 The abnormal high concentration of serum lipids in the diabetic subject is mainly due to increase in the mobilization of free fatty acids from the peripheral fat depots, since insulin inhibits the hormone sensitive lipase. Hypercholesterolemia and hypertriglyceridemia have been reported to occur in diabetic rats33,34 and significant increase observed in our experiment was in accordance to these studies. The marked hyperlipidaemia that characterize the diabetic state may therefore be regarded as a consequence of the uninhibited actions of lipolytic hormones on fat depots.35
The antihyperlipidaemic effect of byesukar may be due to the down regulation of NADPH and NADH, a cofactor in the fat metabolism. Higher activity of glucose-6-phosphatase provides H+ which binds with NADP+ in the form of NADPH and is helpful in the synthesis of fats from carbohydrates. When glycolysis slows down because of cellular activity, the pentose phosphate pathway still remain active in liver to breakdown glucose that continuously provides NADPH which converts acetyl radicals into long fatty acid chains. Byesukar may be capable of oxidizing NADPH. Enhanced hexokinase activity in byesukar treated rats suggests greater uptake of glucose from blood by the liver cells.
Activities of enzymes suggest that enhanced lipid metabolism during diabetes is shifted towards carbohydrate metabolism and it enhances the utilization of glucose at the peripheral sites. One of the possible actions of byesukar may be due to its inhibition of endogenous synthesis of lipids.
Metabolic aberration in diabetic rats suggests a high turnover of triglycerides and phospholipids. Byesukar may antagonize the metabolic aberration and thereby restore the normal metabolism by tilting the balance from high lipids to high carbohydrate turn over. Alteration of fatty acid composition by increased lipid levels contribute to lowering the resistance of tissues and higher rate of oxidative stress. Decreased activity of glucose 6-phosphatase through pentose phosphate shunt results in high reduced glutathione to oxidized glutathione ratio (GSH/GSSG),36 which is coupled with conversion of NADPH to NADP. Byesukar may produce high NADP+ which results in down regulation of lipogenesis and lower risk of the tissues for oxidative stress and high resistance for diabetes.
Thus, our results conclude that byesukar controls the increase in the concentration of blood glucose by increasing glycolysis and decreasing gluconeogenesis. Moreover byesukar treatment significantly reduces the levels of serum and tissue lipids, which are actively raised in alloxan diabetic rats. This could be due to different types of active principles, each with a single or diverse range of biological activities. Cassia auriculata, one of the main constituent of byesukar has been reported to possess pharmacologically active component, di-(2-ethyl) hexyl phthalate.37Eugenia jambolana has been reported to contain flavanol such as myricetin 3-o-(4"- acetyl)-alpha-L-rhamnopyranoside.38 Similarly Thespesia populnea has been reported to possess pharmacologically active sesquiterpenoids.39 Further biochemical and pharmacological investigations are in progress to elucidate in detail the mechanism of action of this drug.