Background and objective. Prophylaxis therapy is indicated in cirrhotic patients with large esophageal varices or small varices with red wale signs (high risk esophageal varices; HREV). Endoscopic surveillance to detect HREV is currently recommended. The objective of this study is to identify non-invasive predictors of HREV in cirrhotic patients.
Design and methods. Adult cirrhotic patients without previous variceal bleeding were prospectively included. All patients underwent a complete biochemical workup, upper digestive endoscopy, and ultrasonographic measurement of spleen bipolar diameter. Platelet count/spleen diameter ratio (PC/SD) was calculated for all patients. The association of these variables with the presence of HREV in upper endoscopy was tested using univariate and multivariate analysis. Receiver operating characteristic (ROC) curves were constructed for variables associated with HREV.
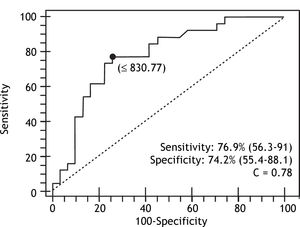
Results. Sixty-seven patients were included. The prevalence rate of HREV was 50%. Age, gender (female), platelet count, spleen diameter, PC/ SD ratio, total bilirrubin, prothrombin activity (INR), Child-Pugh score, clinical and ultrasonographic ascites were significantly associated with presence of HREV in univariate analysis. Age and PC/SD ratio were the parameters independently associated with HREV in a multivariate analysis, with OR 8.81 (CI 95%: 1.7-44.9) and OR 11.21 (CI 95%: 2.8-44.6) respectively. A PC/SD ratio cut-off value under 830.8 predicted HREV with 76.9% sensitivity, 74.2% specificity and 77.8% negative predictive value (ROC curve area: 0.78).
Conclusions. The PC/SD ratio was significantly associated with HREV, but with suboptimal sensitivity and specificity. Therefore, the results of this study do not support the routine clinical use of PC/SD ratio for screening of HREV.
The development of esophageal varices in patients with liver cirrhosis is a common complication. The prevalence of esophageal varices among these patients may range from 60 to 80%.1-4 Variceal bleeding occurs in 20-40% of patients with varices and the reported mortality associated with a variceal bleeding episode is 20-35%.4
In 2005, the Baveno IV consensus stated that cirrhotic patients with portal hypertension should have endoscopic screening for esophageal varices at diagnosis.5 Patients with large esophageal varices or varices with red wale signs are considered high risk esophageal varices (HREV) and they should begin primary prophylaxis for variceal bleeding.6 The use of non-selective beta blockers or band ligation in patients with HREV can reduce the incidence of variceal bleeding in approximately 50%.7 Other authors have suggested that patients with small esophageal varices without risk factors (red wale signs, Child C) should repeat the endoscopy at 1-2 year intervals, at 2-3 year intervals in patients without varices and compensated cirrhosis, and at 1 year in patients without varices and decompensated cirrhosis to evaluate esophageal variceal progression and, according to the findings the clinician should initiate primary prophylaxis when indicated.7-8
In order to reduce the increasing burden of endoscopic units, some studies have attempted to identify variables that non-invasively predict the presence of esophageal varices. These studies have shown that clinical, biochemical and ultrasonographic parameters are associated with presence of esophageal varices.9-24 Most of the reported variables are directly or indirectly associated with portal hypertension, such as decreased platelet count, splenomegaly and ascites. However, in patients with liver cirrhosis, the presence of decreased platelet count can be associated with several factors unrelated with portal hypertension, such as shortened platelets mean half life, decreased thrombopoietin production, or mielotoxic effects of alcohol.25 On the other hand, the presence of splenomegaly in cirrhotic patients is likely the result of vascular disturbance that are mainly linked to portal hypertension.26 Overall, no variable alone have enough power to assess the presence of esophageal varices without upper endoscopic study.
Remarkable results were reported by Giannini, et al. using platelet count/ultrasonographic spleen diameter ratio (PC/SD ratio) as a parameter linking thrombocytopenia to spleen size in order to introduce a variable that takes into account decreased platelet count which most likely depends on hypersplenism caused by portal hypertension.27 In this study, the PC/SD ratio was independently associated with the presence of esophageal varices with 100% sensitivity and 77% specificity.
There are several studies identifying non-invasive variables that predict the presence of esophageal varices.8-23 However, there are no previous studies assessing the utility of clinical, biochemical and ultrasonographic variables, including PC/SD ratio, as non-invasive predictors of HREV.
The objective of the current study is to identify non-invasive predictors of HREV in cirrhotic patients.
Patients and MethodsAdult cirrhotic patients referred to the gastroenterology outpatient clinic of the Pontificia Universidad Católica de Chile in Santiago, were prospectively included, between December 2004 and July 2007. Unstable patients, particularly those with acute variceal bleeding at admission, were excluded from this study. Patients with previous variceal bleeding, sclerosis or band ligation of esophageal varices, transjugular intrahepatic portosystemic stent shunt (TIPS) or surgery for portal hypertension were also excluded.
All patients underwent complete clinical and biochemical examination, abdominal ultrasound and upper endoscopic evaluation for esophageal varices. Cirrhosis was diagnosed by means of laboratory, radiological and physical examination findings, or by liver histology in the case of absence of clear clinical signs of liver cirrhosis. Clinical data included age, gender, etiology of cirrhosis and medication use (beta-blockers, diuretics or nitrites). Physical exam findings included splenomegaly, ascites and hepatic encephalopathy. Laboratory data included bilirru-bin, albumin, creatinine, ASAT, ALAT, prothrombin activity expressed as International Normalized Ratio (INR) and platelet count. Patients were classified according to Child-Pugh score28 and Model of End Stage Liver Disease (MELD) score.29 All the ultrasonographic studies were performed by one experienced operator with a Hitachi EUB-525 equipment. The presence of ascites and the maximum spleen bipolar diameter expressed in millimeters were estimated by means of ultrasound scan. Endoscopies were performed in two endoscopic units using a video endoscope (FujinonTM EG-S90WR). Esophageal varices were classified according to AASLD practice guidelines criteria (no varices, small varices and large varices).8 HREV included large varices with or without red signs and small varices with red signs (red wale marks, cherry-red spots, hematocys-tic spots or diffuse erythema).30
Statistical analysisAge, gender, Child-Pugh score, MELD score, clinical and ultrasonographic ascites, platelet count, albumin, total bilirrubin, ASAT, ALAT, ultrasonographic spleen diameter, platelet count/ultrasonographic spleen diameter ratio (PC/SD ratio) were the parameters included in the statistical analysis. The Mann-Whitney U test was used for comparison of quantitative variables while qualitative variables were compared using the χ2 test. A multivariate analysis with logistic regression model was performed on parameters which were significantly different in the univariate analysis between patients with or without HREV, in order to determine the variables independently associated with the presence of HREV. Data were shown as mean (range) or value and 95% confidence interval (95% CI). For all analyses a p value < 0.05 was considered statistically significant.
Receiver operating characteristic (ROC) curves31 were constructed to find the best sensitivity and specificity cut off values of the significant variables for the presence of HREV. The validity of the model was measured by means of the concordance (c) statistic (equivalent to the area under the ROC curve). A model with a c value above 0.7 is considered useful while a c value between 0.8 and 0.9 indicates excellent diagnostic accuracy.32 Data were analyzed using the SPSS package for Windows (SPSS Inc., Chicago, Illinois, USA).
ResultsA total of 67 patients were included in this study. The average age was 66 ± 12.2 years (Mean ± SD). Gender distribution was: male 29 (43.3%) and female 38 (56.7%). Thirty-one (46.2%) patients were Child-Pugh class A, twenty-six (38.8%) were class B and ten (15%) were class C. All patients included underwent upper digestive endoscopy, and 57 (85%) of them had endoscopic evidence of esophageal varices. Thirty three patients out of 57 patients with esophageal varices had HREV (57.9%). On the other hand, 10 patients had absence of esophageal varices (14.9%). The etiology of cirrhosis was hepatitis virus infection in 7.5%, alcohol abuse in 26.9%, autoimmune hepatitis in 11.9%, primary biliary cirrhosis in 14.9%, non-alcoholic steatohepatitis in 14.9% and cryptogenic or not determined in 26.9%.
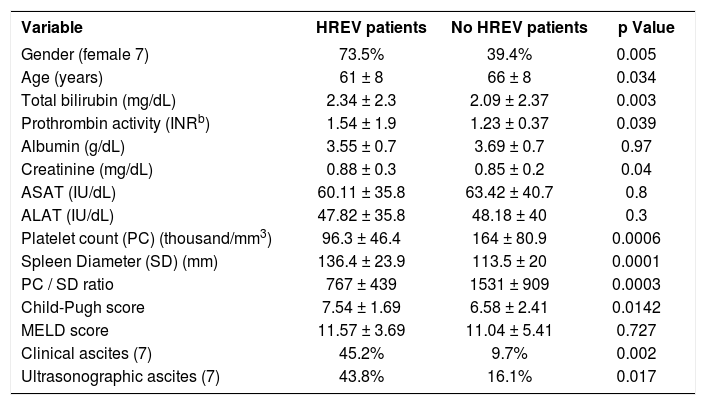
Clinical, laboratory, ultrasonographic data and results of univariate analysis are shown in Table 1. HREV patients were younger than no HREV patients (61.4 ± 8.4 v/s 66.2 ± 8.9; p = 0.034). HREV patients showed a higher proportion of females 25/34 (73.5%) compared with no HREV patients 13/33 (29.4%) with a p value = 0.005. Higher total bilirrubin (2.34 ± 2.3 v/s 2.09 ± 2.37; p=0.003) and Child-Pugh Score (7.54 ± 1.69 v/s 6.58 ± 2.41; p= 0.01) was observed among HREV patients. A higher proportion of clinical ascites (45.2% v/s 9.7%; p = 0.02) and ultrasonographic ascites (43.8% v/s 16.1%; P = 0.017) were observed in HREV patients compared with no HREV patients.
Comparison of clinical, laboratory and ultrasonographic data of cirrhotic patients divided according to the presence of high risk esophageal varices (HREV).
| Variable | HREV patients | No HREV patients | p Value |
|---|---|---|---|
| Gender (female 7) | 73.5% | 39.4% | 0.005 |
| Age (years) | 61 ± 8 | 66 ± 8 | 0.034 |
| Total bilirubin (mg/dL) | 2.34 ± 2.3 | 2.09 ± 2.37 | 0.003 |
| Prothrombin activity (INRb) | 1.54 ± 1.9 | 1.23 ± 0.37 | 0.039 |
| Albumin (g/dL) | 3.55 ± 0.7 | 3.69 ± 0.7 | 0.97 |
| Creatinine (mg/dL) | 0.88 ± 0.3 | 0.85 ± 0.2 | 0.04 |
| ASAT (IU/dL) | 60.11 ± 35.8 | 63.42 ± 40.7 | 0.8 |
| ALAT (IU/dL) | 47.82 ± 35.8 | 48.18 ± 40 | 0.3 |
| Platelet count (PC) (thousand/mm3) | 96.3 ± 46.4 | 164 ± 80.9 | 0.0006 |
| Spleen Diameter (SD) (mm) | 136.4 ± 23.9 | 113.5 ± 20 | 0.0001 |
| PC / SD ratio | 767 ± 439 | 1531 ± 909 | 0.0003 |
| Child-Pugh score | 7.54 ± 1.69 | 6.58 ± 2.41 | 0.0142 |
| MELD score | 11.57 ± 3.69 | 11.04 ± 5.41 | 0.727 |
| Clinical ascites (7) | 45.2% | 9.7% | 0.002 |
| Ultrasonographic ascites (7) | 43.8% | 16.1% | 0.017 |
HREV: High Risk Esophageal Varices. INR: International Normalized Ratio. MELD: Model of End Stage Liver Disease. Data is expressed in mean (± Standard Deviation) or percentage. Univariate analysis with Mann-Whitney U test and χ2.
Platelet count was significantly lower among patients with HREV (96.3 ± 46.4 v/s 164 ± 80.9; p = 0.0006). Larger spleen diameter was observed in HREV patients compared with no HREV patients (136.4 ± 23.9 v/s 113.5 ± 20; p = 0.0001). Finally, the PC/SD ratio in patients with HREV was significantly lower compared with no HREV patients (767 ± 439 v/s 1531 ± 909; p = 0.0003).
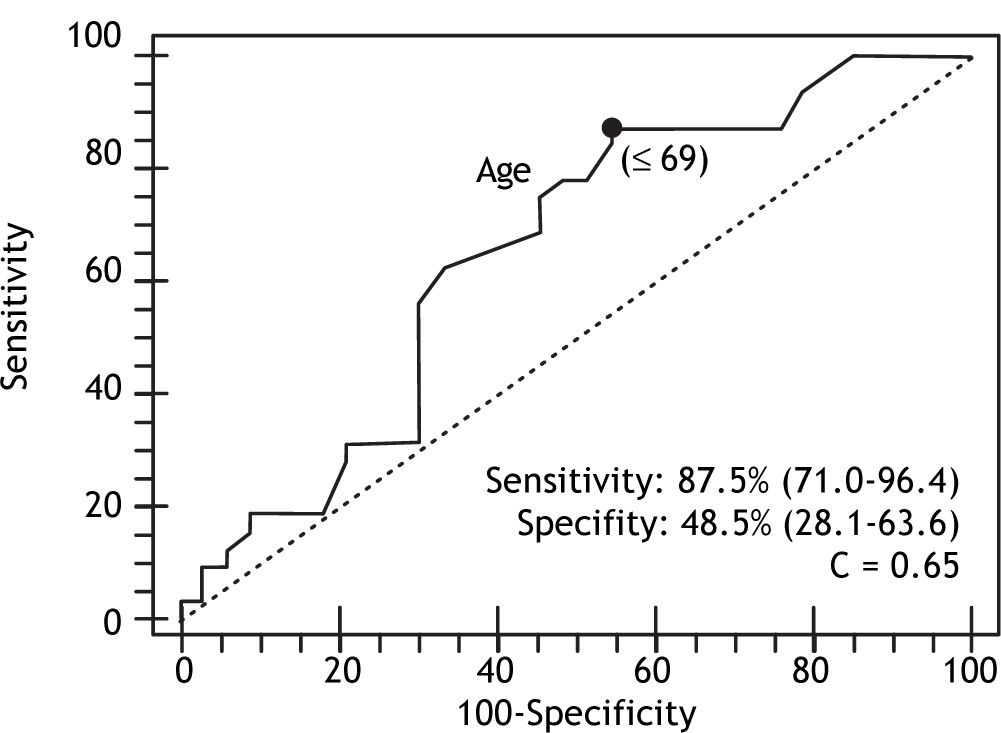
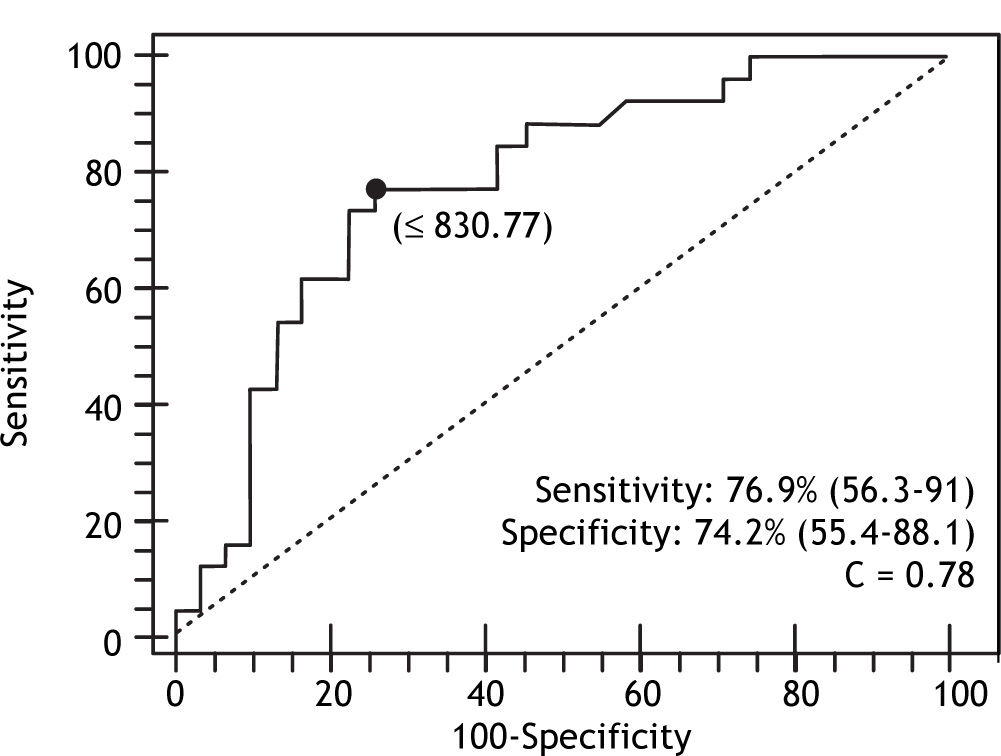
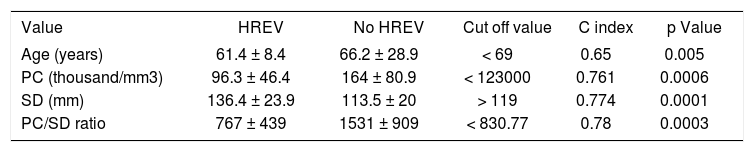
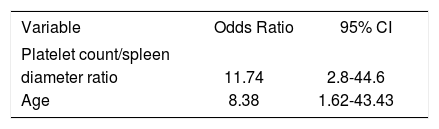
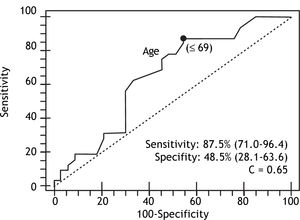
In the multivariate analysis with logistic regression, only age and PC/SD ratio variables were independently associated with the presence of HREV. ROC curves were constructed in order to find the best sensitivity and specificity cut off value for the variables independently associated with the presence of HREV in the multivariate analysis. Age cut off point was < 69 years with sensitivity 87.5% (CI 95% = 71.0-96.4) and specificity 48.5% (CI 95% = 28.1-63.6); and C= 0.65 expressing the area under the ROC curve (Figure 1 and Table 2). The odds ratio (OR) was 8.38 (CI 95% = 1.62-43.43) calculated for the referred cut off point. The PC/SD ratio cut off point was < 830.8 with sensitivity of 76.9% (IC 95% = 56.3-91%) and specificity 74.2% (IC 95% = 55.4-88.1%); and C= 0.78 (Figure 2 and Table 3). The Odds Ratio (OR) was 11.74 with a confidence interval (CI) 95% = 2.8-44.6 for the referred cut point (Table 3). The PC/SD ratio showed a positive likelihood ratio (LR) of 2.98 and negative LR of 0.31. PC/SD ratio below 830.8 had a positive predictive value of 71.4%. On the other hand, the negative predictive value was 77.8%.
Age, platelet count, spleen diameter and platelet count/spleen diameter ratio of the cirrhotic patients subdivided according to the presence of HREV.
| Value | HREV | No HREV | Cut off value | C index | p Value |
|---|---|---|---|---|---|
| Age (years) | 61.4 ± 8.4 | 66.2 ± 28.9 | < 69 | 0.65 | 0.005 |
| PC (thousand/mm3) | 96.3 ± 46.4 | 164 ± 80.9 | < 123000 | 0.761 | 0.0006 |
| SD (mm) | 136.4 ± 23.9 | 113.5 ± 20 | > 119 | 0.774 | 0.0001 |
| PC/SD ratio | 767 ± 439 | 1531 ± 909 | < 830.77 | 0.78 | 0.0003 |
Cut off value were identified by ROC curves. C index: Area under the ROC curve. PC: Platelet Count. SD: Spleen Diameter. Values expressed in mean (± Standard Deviation). Statistical analysis was carried out with Mann-Whitney U test or χ2
Multivariate analysis of variables associated with presence of HREV.
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| Platelet count/spleen | ||
| diameter ratio | 11.74 | 2.8-44.6 |
| Age | 8.38 | 1.62-43.43 |
The multivariate analysis included the following variables: gender, age, platelet count, spleen diameter, Child-Pugh score, clinical ascites, ultrasonographic ascites and platelet count/spleen diameter ratio.
There is an increasing demand of endoscopic studies, and capacity to fulfill them is limited. Endoscopic screening for HREV every one or two years is difficult to achieve. Searching for non-invasive parameters associated with high risk esophageal varices aims for a screening method that reduce endoscopic requirement and public costs. These parameters could detect patients at particular risk of HREV, and select them to have their endoscopic surveillance before patients with lower risk. In this study we evaluate clinical, laboratory and ultrasonographic parameters previously described in the literature associated with presence of esophageal varices. Although previous studies have evaluated this correlation showing promising results, many of them consider only presence or absence of esophageal varices. The study by Gianini, et al. incorporated the platelet count/ultrasonographic spleen diameter ratio with interesting results (100% sensitivity and 77% specifity). The PC/SD ratio was validated in a multicenter study with 91.5% of sensitivity and 67% of specifity33 and a second validation in a different group of patients was carried out with similar results.34 However, in a recent study carried out by Berzigotti, et al.35 no independent association of spleen diameter or platelet count was demonstrated.
The current study focused on parameters associated with HREV in order to select patients to begin primary prophylaxis with beta blockers. The variables included were most relevant parameters detected in previous studies. The results showed significant association of several variables [age, gender (female), platelet count, spleen diameter, platelet count/spleen diameter ratio (PC/SD) ratio, total bili-rrubin, Child-Pugh score, clinical and ultrasonographic ascites] with HREV in univariate analysis, but only two variables were significant in multivariate analysis: PC/SD ratio and age. The only parameter that had adequate area under ROC curve within useful range was PC/SD ratio and was close to excellent diagnostic accuracy (C index: 0.78). This ratio combines two variables associated with portal hypertension and correlated with esophageal varices in previous studies. However, the specificity and sensitivity of the cut off value were not good enough to replace endoscopic surveillance of HREV (74.2% and 76.9% respectively). The association of platelet count and spleen diameter with presence of large esophageal varices, which is one of the endoscopic findings of patients with HREV, was confirmed in a recent study by Sharma S, et al.36
The variability of the parameters values found in HREV and non HREV patients was very important. This finding could be explained by the variability of platelets count and the umltiple factors that influence them (platelet mean half life, bone marrow depression, medications, etc). Changing the cut off point for maximum sensitivity (moving the cut off point to the right in ROC curve) reduce dramatically the specificity of the PC/SD ratio, making not practical to use this parameter for screening. We built ROC curves for platelet count and ultrasonographic spleen diameter. Specificity for each variable was lower than the specificity observed for the combined PC/ SD ratio (platelet count cut off value= 123,000/uL, sensitivity = 84.6%, specificity = 66.7%, C= 0.761; ultrasonographic spleen diameter cut off point= 119 mm, sensitivity= 75.8%, specificity= 68.7%, C= 0.774).
The mean value of PC/SD ratio was significantly lower among patients with HREV compared with patients without HREV (767 ± 439 vs. 1531 ± 909) p= 0.0003. Gianini, et al. found a cut off value of 909 PC/SD ratio in order to rule out presence of esophageal varices in patients with cirrhosis. In the present study we could not find a cut off point in order to rule out patients with very low probability of HREV.
The results of the current study do not support the use of PC/SD ratio for screening of HREV. Periodic endoscopy should not be replaced by the parameters included in this study for HREV screening. PC/SD ratio represents an initial approach to predict the presence of HREV, but endoscopic screening must be used in every cirrhotic patient to detect HREV in order to select patients for primary prophylaxis.