The patient was a 43-year-old man with chronic hepatitis B without history of hepatocellular carcinoma (HCC), who was first diagnosed with thrombosis in right portal vein trunk and portal vein branches and ruptured esophageal varices in October 2011. He underwent endoscopic variceal ligation, but ruptured repeatedly. Despite anti-coagulant therapy, the thrombosis expanded from right portal vein trunk to upper mesenteric vein in March 2012. Computed tomography (CT) scan showed that portal vein thrombosis had low density from early to late phase. No focal liver lesions were identified by CT scan or ultrasound, and alpha-fetoprotein (AFP) was within normal range. He died by intractable esophageal variceal bleeding in April 2012. Pathological examination of autopsy specimen showed that portal vein thrombosis was consistent with poorly-differentiated HCC. The portal vein tumor thrombosis (PVTT) had only a few tumor vessels, which were compressed by fibromatous change originating from HCC formation, so were represented as low-density lesions from arterial to portal phase of CT. In addition, PVTT was negative for AFP, so representing serum value of AFP within normal range. PVTT had positive staining for c-kit, which is a liver stem cell marker. Liver tumors in the whole liver parenchyma were not found pathologically. PVTT might have the characteristics of presumed liver cancer stem cells. We experienced the first case of HCC only in portal vein without liver parenchyma tumor nodules, with difficult differential diagnosis from a non-malignant portal vein thrombosis. We also reported new tumor profiles of the portal venous tumor growth- type of HCC.
Recent progress in imaging techniques has permitted the diagnosis of hepatocellular carcinoma (HCC) at an early stage. However, portal venous invasion is still found in 12.5–39.7% of patients with HCC.1’2 According to the 16th National Survey for Primary Liver Cancer in Japan, 808 of 5,130 patients (16%) who received hepatic resection had macroscopic portal venous invasion.3 All of these HCC patients with portal venous invasion had large or multiple tumors in the liver parenchyma, and had a high level of serum alpha-fetoprotein (AFP).4 By computed tomography (CT) scan, portal venous tumor thrombosis (PVTT) was found to have a high density in the arterial phase and a low density in the portal phase.5 In our case, however, no focal liver lesions were identified by CT scan and ultrasound (US), and AFP was within the normal range. There was no history of HCC. The lesion was only detected in the portal vein, and had a low density from the arterial to portal phase by CT scan. Therefore, the portal lesion was misdiagnosed as portal venous thrombosis, but diagnosed as HCC by autopsy.
There had been no reports concerning HCC cases only in the portal vein with no focal lesions in the whole liver parenchyma and no history of HCC. In this report, we describe the clinical course and tumor profiles, such as tumor differentiation and immunohistochemical findings, of the portal vein tumor growth-type of HCC.
Case ReportThe patient was a 43-year-old man who had hematemesis. He did not drink alcohol or smoke. He had had chronic hepatitis B after birth, and did not have any other past history including HCC. His mother had chronic hepatitis B. He was followed-up by biannual blood tests and abdominal US or CT from January 2000 in a neighborhood clinic. He suddenly had hematemesis in October 2011 and was diagnosed with ruptured esophageal varices by upper endoscopy. He was admitted to our hospital to stop the bleeding. He underwent endoscopic variceal ligation (EVL). On admission, he did not have edema of lower extremity, flapping tremor, vascular spider, palmar erythema, gynecomastia, and fetor hepaticus. The blood test results on admission were as follows:
- •
White blood cells: 6,400/μΚ
- •
Hemoglobin: 11.8 g/dL.
- •
Platelet count: 23.3 x 104/μΚ
- •
Prothrombin time: 90.0%.
- •
C-reactive protein: 11.5 mg/dL.
- •
Albumin: 3.3 g/dL.
- •
Total bilirubin: 1.3 mg/dL.
- •
Aspartate aminotransferase (AST): 49 IU/L.
- •
Alanine aminotransferase (ALT): 46 IU/L.
- •
Lactate dehydrogenase: 280 IU/L.
- •
Alkaline phosphatase (ALP): 1,454 IU/l.
- •
γ-glutamyltranspeptidase: 318 IU/l.
- •
Choline esterase: 135 IU/l.
- •
HBs Ag: 2,124( + ).
- •
HCV Ab: (−).
- •
AFP: 14 ng/mL.
- •
AFP-lectin fragment 3: 0.0%.
- •
Carcinoembryonic antigen: 1.5 ng/mL.
- •
Carcinogenic antigen 19-9: 35 U/mL.
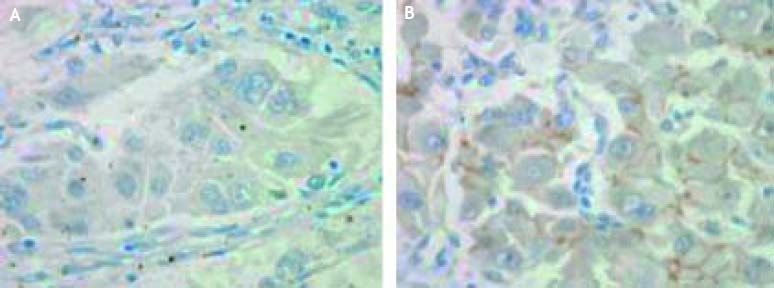
Abdominal dynamic CT scan showed thrombosis in the right portal vein trunk and portal vein branches, which was not observed in April 2011. The thrombosis had a low density from the arterial to portal phase by CT scan (Figures 1A, 1B). Abdominal US showed that the thrombosis was hypo-echoic and lacked a Doppler signal, and the diameter of the right portal vein trunk was slightly enlarged up to 16 mm (Figure 1C). Thickening of gall bladder wall and splenomegaly were also found by CT scan. In addition the liver surface was slightly irregular, and bilateral hepatic lobe was normal in size by CT scan. No focal lesions were found in the liver parenchyma by CT and US. Our case did not have local inflammatory diseases, such as pancreatitis and cholangitis, and past history of traumatic injury, which were thought to be the causes of portal vein thrombosis. Anti-coagulant therapy was performed by drip intravenous injection of heparin in October 2011 and oral administration of warfarin from November 2011. However, the CT scan showed that the thrombosis had expanded from the right portal vein trunk to the upper mesenteric vein in March 2012. The patient underwent EVL against the esophageal variceal bleeding a total of 10 times from October 2011 to April 2012, but the EVL did not stop the bleeding. He died of loss of a large volume of blood and was subsequently subjected to an autopsy. A tissue specimen subjected to hematoxylin and eosin staining showed that portal vein thrombosis was consistent with poorly-differentiated HCC (Figure 2A). By Elastica van Gieson staining, it was shown that HCC raised intra-luminally with no extra-luminal invasion (Figure 2B). The HCC was found from the portal vein trunk to the portal vein branches and upper mesenteric vein. The HCC had only a few tumor vessels, which were compressed by fibromatous change originating from HCC formation (Figure 2C). As for immunohistochemical examinations, HCC was negative for AFP (Figure 3A), and was positive for c-kit (Figure 3B). Tumor nodules in the whole liver parenchyma were not found pathologically (data not shown).
Abdominal dynamic CT and US of portal lesion without focal liver lesions. Abdominal dynamic CT showed that the portal lesion was in the right portal vein trunk and portal vein branches. The lesion had a low density from (A) arterial to (B) portal phase by CT scan. (C) Abdominal US showed that the portal lesion was hypo-echoic and lacked a Doppler signal, and the diameter of the right portal vein trunk was slightly enlarged up to 16 mm. No focal liver lesions were found by CT and US.
Histopathological findings of portal lesion from autopsy specimen. The tissue specimen obtained at autopsy showed that the portal lesion was consistent with poorly-differentiated HCC (A. Hematoxylin and eosin staining, x 200). The HCC raised intra-luminally with no extra-luminal invasion (B. Elastica van Gieson staining, x 12.5). The HCC was found from the portal vein trunk to the portal vein branches and upper mesenteric vein. From these pathological findings, we diagnosed PVTT. The HCC had only a few tumor vessels (arrow), which were compressed by fibromatous change originating from HCC formation (C. Hematoxylin and eosin staining, x 12.5).
This case posed a diagnostic dilemma. This patient had chronic hepatitis B, a clear risk factor for HCC and PVTT. However, no liver parenchyma tumor nodules were identified by ultrasound and CT scan and the AFP was within the normal range. This patient also had no history of HCC. Moreover, the portal lesion had a low density from the arterial to portal phase by CT scan. These findings were suggestive of portal venous thrombosis. Portal venous thrombosis is considered a rare clinical and pathological entity.6 Thrombus formation usually begins in the portal vein and sometimes extends to other branches of the portal system such as splenic vein and mesenteric vein.7,8 Portal venous thrombosis is also associated with hepatitis B virus infection.9
PVTT is the most common vascular invasion in HCC1 and is closely related to intrahepatic recurrence.10 This kind of PVTT is known to be a direct extension of liver parenchyma tumor into the main portal trunk and its branches.11 It has already been reported that the PVTT has a similar expression profile to liver parenchyma tumor nodules as detected using a cDNA array,12 which indicates that they have similar biological characteristics.
On the other hand, PVTT is rarely observed to be distant from liver parenchyma tumor nodules.13 Previous studies have shown that there are distinct biological characteristics between PVTT and liver parenchyma tumor nodules.14 It has been shown that PVTT has marked expression of c-kit, which is a liver stem cell marker, but liver parenchyma tumor does not, indicating that they might have different origins.14 In this patient, although liver parenchyma tumor was not found in the whole liver, PVTT showed c-kit expression. PVTT might have the characteristics of presumed liver cancer stem cells, not mature hepatocytes. Hepatic oval cells might be the origin of PVTT. First, oval cells expressed c-kit.15 Second, oval cells were observed at the periportal area and in the surrounding parenchyma.16 PVTT might have different molecular signatures and show a more malignant phenotype, although there are still many potential biomarkers left unidentified.
In this patient, PVTT expressed c-kit, but did not express AFP, which is also a liver stem cell marker. Serum AFP level was also within the normal range. A previous study showed that AFP elevation developed early in the carcinogenic regime, preceding formation of foci, and it was shown not to be expressed by proliferating oval cells.17 Therefore, it was speculated that the AFP level would return to normal in this case. In addition, the expression of c-kit is closely associated with tumor differentiation.18 Most of the cells are poorly differentiated.18 In our case, PVTT consisted of poorly- differentiated HCC. Many cases of HCC have been reported to show hypovascularity.19 This patient had only a few vessels in the PVTT pathologically. In addition, the vessels were compressed by fibromatous change originating from HCC formation. Therefore, it was thought that the PVTT was not enhanced from the arterial to portal phase by CT scan.
In our case, the portal vein lacked a Doppler signal, and the diameter of the right portal vein trunk was slightly enlarged as shown by abdominal US. Thickening of gall bladder wall and splenomegaly were also found by CT scan. These findings were reported to be related to portal hypertension.7 PVTT might decrease portal flow and lead to portal hypertension, resulting in bleeding of esophageal varices.
In conclusion, our case is the first for which HCC only in the portal vein without liver parenchyma tumor nodules has been reported. Our case is a rare example of PVTT with difficult differential diagnosis from non-malignant portal vein thrombosis. In addition, our case is the first for which the tumor profiles, such as poor differentiation and c-kit expression, have been reported in the portal venous tumor growth- type of HCC.
Conflict of InterestWe declare that we have no conflicts of interest.
Financial SupportNone.
AcknowledgmentsWe thank the nurses and our colleagues in the Department of Gastroenterology and the Gastroenterology Ward at Kobe University Hospital for their cooperation. This work was supported by Grant-in-Aid for Research Activity Start-up [M.S], and was also supported by grants for the Global COE Program, Global Center of Excellence for Education and Research on Signal Transduction Medicine in the Coming Generation from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [M.Y. and T.A.].