Background. Hepatocellular carcinoma (HCC) is the most common malignancy that develops in cirrhotic livers. Its clinical and epi-demiological characteristics and mortality rates vary according to geographical region. The objective of this study was to evaluate the clinical profile, epidemiological characteristics, laboratory parameters, treatment and survival of patients with HCC.
Material and methods. Patients with HCC seen between 2000 and 2012 were studied. The Kaplan-Meier method was used for survival analysis according to variables in question.
Results. The study included 247 patients with a mean age of 60 ± 10 years. There was a predominance of males (74%). The main etiologies of HCC were HCV infection (55%), excessive alcohol consumption (12%), and HBV infection (8%). Liver cirrhosis was present in 92% of cases. The mean tumor number and diameter were 2 and 5 cm, respectively. Patients meeting the Milan criteria corresponded to 43% of the sample. Liver transplantation was performed in 22.4% of patients of the Milan subset and in 10% of the whole sample. The overall mean survival was 60 months, with a 1-, 3- and 5-year survival probability of 74%, 40% and 29%, respectively. Lower survival was observed among patients with alcoholic etiology. Survival was higher among patients submitted to liver transplantation (P < 0.001), TACE (P < 0.001), or any kind of treatment (P < 0.001). However, no difference was found for surgical resection (P = 0.1) or sorafenib (P = 0.1).
Conclusion. Patients with HCC were mainly older men diagnosed at an advanced stage. Treatment was associated with better overall survival, but few patients survived to be treated.
Hepatocellular carcinoma (HCC) is the sixth most common tumor in the world and its incidence and risk factors vary widely.1 This tumor is three-fold more common in men than in women and affects individuals at a mean age of 64 years.2 Liver cirrhosis is the main risk factor for the development of HCC.3,4 The most frequent etiologies are hepatitis C infection in the United States,3,5 Europe and Japan, and hepatitis B infection in Asia and Af-rica.6 Mortality from HCC is high, with an estimated 600,000 deaths per year worldwide.6
In Brazil, the incidence of HCC ranges from 3.3% to 6% per 100,000 persons and mortality rates range from 3.6% to 6% per 100,000 persons per year.1 Data from a Brazilian national survey indicate a predominance of the disease in men older than 50 years with cirrhosis and HCV infection. However, these findings differ between the different regions of the country, especially between the North/Northeast and South/Southeast.7 Moreover, there is a paucity of data from these regions regarding diagnosis, type of study population (from private or public services), and treatment. Imaging equipment is critical for the diagnosis of HCC and is rare in Brazilian public hospitals. In addition, liver transplantation (LT) is only available in large Brazilian cities. Therefore, more studies on HCC are needed to elaborate diagnostic and treatment strategies for this population. The aim of this study was to evaluate the clinical profile, epidemiological characteristics, laboratory parameters, treatment and survival of patients with HCC seen at a specialized public center in Brazil.
Material and MethodsWe studied patients with HCC seen between 2000 and 2012 at a specialized center of the Federal University of São Paulo, São Paulo, Brazil. The Ethics Committee of the Federal University of São Paulo approved the study.
At the time of diagnosis, the patients were investigated for clinical, demographic, laboratory, and radiological features. Differences in survival were analyzed according to etiology and to the different therapeutic modalities used. For survival analysis, follow-up was censored on December 30, 2012. Data regarding death were obtained from the hospital records in more than 90% of cases. In some cases, this information was obtained by contacting the family.
Clinical and laboratory assessmentClinical evaluation consisted of a detailed clinical history and physical examination. The laboratory tests included serum measurement of albumin (g/dL), alpha-fetoprotein (AFP, IU/mL), aspartate aminotrans-ferase (AST, U/L), alanine aminotransferase (ALT, U/L), alkaline phosphatase (U/L), gamma-glutamyltransferase (GGT, U/L), total bilirubin (mg/dL), international normalized ratio (INR), platelets (n/μL), and creatinine (male < 1.2; female < 0.9 mg/dL).
Viral markers such as HBsAg, anti-HBc, HBeAg, anti-HBe and anti-HCV were assayed using commercial ELI-SA kits. Detection of HBV DNA and HCV RNA was performed by RT-PCR with a detection limit of 20 IU/ mL (Cobas TaqMan HBV, Roche Diagnostics) and 50 IU/ mL, respectively.
Liver cirrhosis was confirmed by histological analysis and/or based on clinical-laboratory parameters such as as-cites, hepatic encephalopathy, portal hypertension (splenomegaly, esophageal varices, thrombocytopenia), and ultrasonographic signs suggestive of liver cirrhosis. Excessive alcohol consumption as a cause of cirrhosis was defined as ethanol consumption > 40 g/day in women and > 60 g/day in men for more than 5 years. Patients with liver cirrhosis were evaluated using the Child-Turcotte-Pugh and MELD scores.
Diagnosis of hepatocellular carcinomaThe diagnosis of HCC was based on triple-phase imaging by computed tomography or magnetic resonance of the liver that showed a hypervascular tumor in the arterial phase (wash in) and washout in the portal or equilibrium phase. Tumors not defined by the imaging exams were submitted to image-guided biopsy. The tumor diameter and number, tumor macrovascular invasion, and extrahe-patic metastases were analyzed by chest/abdominal tomography and bone scanning.
Treatment of hepatocellular carcinomaWhen indicated, treatment consisted of LT, surgical resection, transarterial chemoembolization (TACE), radiof-requency thermal ablation (RFA), percutaneous ethanol injection, and administration of sorafenib. The patients were classified according to the Milan criteria,8 and each therapeutic modality was performed according to the Barcelona Clinic Liver Cancer (BCLC) staging system9 or experience of the service.
Statistical analysisThe results are expressed as the mean ± standard deviation. Survival was defined as the interval between the date of HCC diagnosis and either the date of liver-related death or the last follow-up on December 30, 2012. The Kaplan-Meier method was used for survival analysis. A level of significance of P < 0.05 was adopted. Statistical analysis was performed using the SPSS 16 program (Chicago, IL, USA).
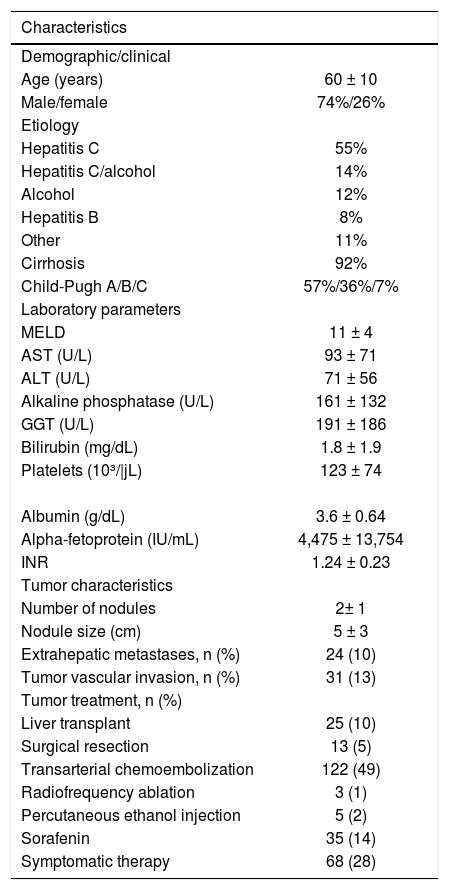
ResultsThere were 247 HCC patients in the study. The mean age of these patients was 60 ± 10 years and there was a predominance of men (74%). The main etiologies were infection with HCV (55%), followed by infection with HCV associated with excessive alcohol consumption (14%), excessive alcohol consumption (12%), infection with HBV (8%), and other causes (11%). Liver cirrhosis was present in 92% of the patients who were classified as Child-Tur-cotte-Pugh A (57%), B (36%), and C (7%) (Table 1).
Demographic, clinical, laboratory and tumor characteristics of patients with hepatocellular carcinoma (n = 247).
| Characteristics | |
|---|---|
| Demographic/clinical | |
| Age (years) | 60 ± 10 |
| Male/female | 74%/26% |
| Etiology | |
| Hepatitis C | 55% |
| Hepatitis C/alcohol | 14% |
| Alcohol | 12% |
| Hepatitis B | 8% |
| Other | 11% |
| Cirrhosis | 92% |
| Child-Pugh A/B/C | 57%/36%/7% |
| Laboratory parameters | |
| MELD | 11 ± 4 |
| AST (U/L) | 93 ± 71 |
| ALT (U/L) | 71 ± 56 |
| Alkaline phosphatase (U/L) | 161 ± 132 |
| GGT (U/L) | 191 ± 186 |
| Bilirubin (mg/dL) | 1.8 ± 1.9 |
| Platelets (10³/|jL) | 123 ± 74 |
| Albumin (g/dL) | 3.6 ± 0.64 |
| Alpha-fetoprotein (IU/mL) | 4,475 ± 13,754 |
| INR | 1.24 ± 0.23 |
| Tumor characteristics | |
| Number of nodules | 2± 1 |
| Nodule size (cm) | 5 ± 3 |
| Extrahepatic metastases, n (%) | 24 (10) |
| Tumor vascular invasion, n (%) | 31 (13) |
| Tumor treatment, n (%) | |
| Liver transplant | 25 (10) |
| Surgical resection | 13 (5) |
| Transarterial chemoembolization | 122 (49) |
| Radiofrequency ablation | 3 (1) |
| Percutaneous ethanol injection | 5 (2) |
| Sorafenin | 35 (14) |
| Symptomatic therapy | 68 (28) |
The mean ± SD, median and range of serum AFP levels were 4,475 ± 13,754, 57 and 1-102,436 IU/mL, respectively. The mean serum levels of AST, ALT, alkaline phosphatase, GGT, platelets, albumin, total bilirubin, cre-atinine and INR were 93 U/L, 71 U/L, 161 U/L, 191 U/L, 123,000/μL, 3.6 g/dL, 1.8 mg/dL, 1.0 mg/dL and 1.24, respectively. The mean MELD score was 11 (Table 1).
Tumor characteristicsThe mean number of nodules was 2 ± 1 (range: 1-7 nodules) and the mean diameter was 5 ± 3 cm (range: 0.9-21 cm). Extrahepatic metastases and tumor macrovascular invasion were observed in 10% and 13% of cases, respectively. Extrahepatic metastases were detected mainly in regional lymph nodes, lungs, and bone. The number of patients meeting the Milan criteria was 107 (43%) (Table 1).
Tumor treatment- •
Group within Milan criteria (Figure 1). Among patients meeting the Milan criteria (n=107), 56% (60/ 107) were on the liver transplant list. Of these, 40% (24/60) underwent LT, 15% were waiting for LT, and 45% (27/60) left the list. Among the patients not listed (47/107), 4% (2/47) were submitted to RFA, 8% (4/47) to percutaneous ethanol injection, and 17% (8/47) to surgical resection. The remaining patients were not listed because of advanced age, severe comorbidities and tumor progression, or were lost to follow-up.
- •
Group outside Milan criteria (Figure 2). TACE, sorafenib, RFA and surgical resection were offered to patients outside the Milan criteria (140/247). Downstag-ing to within Milan by TACE was achieved in only one patient. The procedure was well-tolerated. Sorafenib was offered to 14% (35/247) of the patients with advanced-stage HCC at a dose of 800 mg/day. Sorafenib combined with TACE was offered to 71% (25/35) of the patients. The mean daily dose of the drug was 630 mg. Side effects were observed in 37% (13/35) of the patients who received the drug. Diarrhea, headache and hand-foot skin reaction were the main adverse events.
At the time the data were censored, 89 (46.4%) patients had died. The overall mean survival was 60 months, with a 1-, 3- and 5-year survival probability of 74%, 40% and 29%, respectively.
A significant difference in survival was observed between etiologies. Survival was lower among patients with alcoholic etiology or alcoholic etiology and HCV infection. Figure 3 shows the differences in survival according to the different etiologies.
Survival probability according to etiology comparing (A) excessive alcohol consumption with HCV infection, (B) excessive alcohol consumption with HBV infection, (C) HCV infection with and without excessive alcohol consumption, (D) HCV infection with HBV infection, and (E) all etiologies.
There was also a significant difference in survival between treatments. Individual analysis of survival according to the different treatments showed higher survival among patients submitted to LT (P < 0.001), TACE (P < 0.001), or any kind of treatment (P < 0.001). However, no difference was found for surgical resection (P = 0.1) or soraf-enib (P = 0.1) (Figure 4).
Survival probability (A) in all patients of the entire cohort, (B) according to the different treatments, (C) according to liver transplantation (LT), (D) according to surgical resection, (E) according to transarterial chemoembolization (TACE), (F) according to systemic therapy (sorafenib), and (G) according to any treatment.
We found a predominance of men in the study and the mean age was 60. These findings were similar to those reported in a Brazilian national survey on the prevalence of HCC.7 However, some differences were observed when compared to other countries. For example, in Asia, the tumor incidence peaks around 60 years and HBV infection predominates.6,10 These findings agree with our study regarding age but not in relation to etiology because we found a predominance of HCV. Another discrepancy is seen in Japan where HCV predominates, while age ranges from 70 to 75 years.10,11 These findings show that epidemiological differences in HCC between regions cannot always be explained by different etiologies.
With respect to etiology, we observed lower survival among patients with a history of alcohol abuse and survival was also lower when alcohol abuse was associated with HCV infection. This fact has also been reported in the study of Bucci et al. in which patients with alcoholic etiology had significantly lower survival than those with HCV infection alone.12
Liver cirrhosis is the main risk factor for HCC3,8,9,13 and was present in 92% of the cases, in agreement with other studies. Another important finding was that most patients with HCC were classified as Child A (57%). HCC with compensated cirrhosis is commonly found in liver cancer patients.14-16
Abnormal AFP serum values were detected in most patients (63%), but 37% had AFP values within the normal range. This was expected because 30-40% of tumors have normal AFP values.6 Moreover, the utility of AFP for the diagnosis of HCC is known to be limited.17-19
With respect to tumor characteristics, the number of patients outside the Milan criteria was high. The number of patients with advanced HCC differs between studies. In an Indian study, 44% of the patients with HCC had advanced disease,20 while in a larger study conducted by Kumar, et al. (2008), there were 82% advanced cases of HCC.21
In the present study, in contrast to the significant number of patients with advanced disease, the frequency of distant metastases (10%) and macrovascular tumor invasion (13%) was low. Addario, et al. (2011) observed distant metastases and tumor macrovascular invasion in 10% of cases.22 In a large American study, Yang, et al. (2014) found distant metastases in 16% of cases.23 Paul, et al. (2009) observed distant metastases in 13% of cases and macrovascu-lar tumor invasion in 40% .20
Treatment of HCC such as LT, RFA and surgical resection were offered to a small proportion of patients. LT was performed in 22.4% of patients within the Milan criteria and in 10% of the whole sample. These findings are consistent with other studies. In the study of Kitisin, et al. (2011), 13% of patients with HCC underwent LT.15 This percentage was only 7% in the study of Yang, et al. (2014).23 These numbers reflect not only the presence of advanced disease, but also the low availability of donor organs. As expected, the survival rate of patients submitted to LT was higher than the survival of patients receiving no transplant.
Surgical resection had no impact on patient survival. In fact, surgical resection was offered to only 13 patients who had advanced disease, which likely explains the findings. In the study of Carrilho, et al. (2010), the rate of surgical resection was 7% .7 Higher rates of 11% and 12% have been reported in other studies.15,23 The use of TACE for some intermediate tumors as downstaging treatment to within Milan criteria may explain in part the small number of surgical resections found here.
Downstaging of patients with HCC outside the Milan criteria using TACE has been associated with high rates of successful downstaging to within Milan criteria but also with post-LT recurrence and survival.24 However, in our study few patients showed a decrease in tumor size to within Milan criteria for subsequent LT. TACE has also been proposed as a bridging treatment to LT because it may improve survival and reduce the recurrence of HCC after LT.25,26 In our study, 27% of the patients who received TACE were on the waiting list for LT. This procedure resulted in a very low rate of dropout and was well tolerated. In addition, better survival was observed among patients who received TACE compared to those who did not. Although RFA has gained widespread use over recent years as an effective procedure, especially for small HCC not amenable to surgical resection, this procedure is not available at our center.
In the present study, 14% of the patients received soraf-enib either as monotherapy or combined with TACE. The drug was well tolerated by most patients. Data indicate that sorafenib might have a beneficial therapeutic effect on advanced-stage HCC27 by inhibiting tumor cell proliferation and tumor angiogenesis. In addition, sorafenib has been shown to increase the rate of apoptosis in a wide range of tumor models, increasing survival.28 However, no association between survival and the use of sorafenib was observed in our study.
The indication of sorafenib is still controversial. Therefore, the number of patients receiving the drug varies widely. Sanyal, et al. (2010) reported that 6% of patients with HCC used sorafenib.29 This percentage was only 3% in the study of Kitisin, et al. (2011).15 These differences suggest that the populations studied differ in terms of the indications and contraindications to drug use.
This study evaluated the treatment of HCC in Brazil. We found that most HCC patients are diagnosed at an advanced stage, a fact that prevents curative treatment. In addition to a late diagnosis, the limited availability of organs and advanced disease restrict the access to LT. These findings and those of similar Brazilian studies highlight the need to intensify the screening of risk patients for HCC in order to identify this cancer early, which should permit to increase the success rates of conventional treatments.
Abbreviations- •
AFP: alpha-fetoprotein
- •
ALT: alanine aminotransferase
- •
Anti-HBc: hepatitis B core antibody
- •
Anti-HBe: hepatitis B e antibody
- •
Anti-HCV: hepatitis C virus antibody
- •
AP: alkaline phosphatase
- •
AST: aspartate aminotransferase
- •
BCLC: Barcelona clinic liver câncer.
- •
CI: confidence interval.
- •
CTP: Child-Turcotte-Pugh.
- •
GGT: gamma-glutamyltransferase.
- •
HBeAg: hepatitis B e antigen.
- •
HBsAg: hepatitis B surface antigen.
- •
HBV: hepatitis B vírus.
- •
HBV DNA: hepatitis B virus DNA.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C vírus.
- •
HCV RNA: hepatitis C virus RNA.
- •
INR: international normalized ratio.
- •
LT: liver transplantation.
- •
MELD: model for end-stage liver disease.
- •
PEI: percutaneous ethanol injection.
- •
RF: radiofrequency ablation.
- •
RT-PCR: reverse transcription polymerase chain reaction.
- •
TACE: transarterial chemoembolization.
- •
TMI: tumor macrovascular invasion.
The authors declare that they have no competing interests.