Given the severe shortage of liver donors, a carefully evaluation and selection of patients who are likely to obtain a significant survival benefit from liver transplantation (LT) is imperative in order to attain successful outcomes. Cardiovascular and respiratory events remain one of the leading causes of non-graft-related death in LT. A variety of pre-existing cardiac and pulmonary disorders are commonly identified in LT recipients, more so than in the general population. Uncertainties regarding the optimal assessment of cardiovascular and respiratory function in potential transplant candidates have produced a wide variation in the clinical care of tjis population. There is still no consensus on which assessment algorithm confers the best outcomes. Once the diagnosis has been established, the prognosis should be estimated for risk stratification and to confirm the candidacy for LT. Additionally, the challenge remains in knowing how cardiac or respiratory derangements in candidates affect the long-term outcome after LT and which is the magnitude of risk that we as physicians are willing to accept. This article discusses the cardiac and pulmonary aspects of liver disease that may impact recipient selection. Relevant literature focused upon the most common entities in this field is presented in this review.
Liver transplantation (LT) has revolutionized the approach of liver failure altering the natural history of end-stage liver disease (ESLD) and is now considered the only definite treatment for patients in whom conventional medical therapy has failed.1 The gradual development of the surgical procedure, patient selection and immunosuppressive therapy have resulted in an overall 1-yr survival rate > 80% in those patients who, otherwise, have little or no hope of survival. As success with LT has increased, the criteria for selection have also changed. The improvement in outcomes has led to a greater demand of LT. Absolute contraindications such as severe cardiovascular or respiratory disease among others were established in the early era of LT and nowadays an increasing willingness to accept patients with a higher risk profile reflects the increased experience and good results in large centers.
Notwithstanding, LT is a high-risk surgery that incurs a significant cost in economic terms and in the preventable loss of human organs. Post-transplant cardiovascular and respiratory complications remain one of the leading causes of non-graft-related death after LT.2,3 Given the severe shortage of donor livers which lags far behind that of the recipients, a careful evaluation and selection of patients who are likely to obtain a significant survival benefit from transplantation is pivotal in order to obtain successful outcomes. Therefore, the pre-LT assessment has two main objectives. First, an individual evaluation and stratification of the associated perioperative risk is essential to exclude a patient who has some comorbidity that increases the inherent surgical risk. Second, estimation of the long-term prognosis considering other potential extra-hepatic disorders in order to establish a realistic risk-benefit ratio. The natural history of the patient’s coexisting disease must be carefully compared with the anticipated survival after LT. Some coexisting cardio-respira-tory diseases may reduce the expected medium and long-term survival and the transplant would not yield adequate benefit from the allograft. Thus, the best evaluation for the individual patient must take into account not only the perioperative risk-benefit ratio of particular cardiopulmonary condition but also how this condition can affect the life expectancy of the patient (Table 1).
Cardio-respiratory coexisting disease in liver transplant candidates. Relevant data and liver transplant impact.
| Prevalence in LT candidates | History | Signs and symptoms | Screening method | Diagnosis | Expected outcome of coexisting disease after LT | |
|---|---|---|---|---|---|---|
| Coronary artery disease | 2.5-27% | Cardiovascular risk factors | Asymptomatic, dyspnea or chest pain | Cardiac stress testing | Selective coronary arteriography | Progression, expected impairment of cardiovascular risk factors |
| Cirrhotic cardiomyopathy | Unknown but expected high | None | Exercise and fluid overload intolerance | Echocardiography | Echographic findings and electrophysiological study | Reversal after LT |
| Valvular heart disease | Unknown but expected low (severe forms) | Previous diagnosis in childhood or by echocardiography | Dyspnea | Echocardiography | Echocardiography | Progression irrespective of LT |
| Chronic obstructive Pulmonary disease | 18% | Smoking | Chronic cough, cyanosis, dyspnea | - | Pulmonary function test | Progression irrespective of LT |
| Hepatic hydrothorax | 5-12% | Refractory ascites | Asymptomatic, dyspnea | - | Chest radiograph | Resolution after LT |
| Hepatopulmonary syndrome | 20% | - | Asymptomatic, spider naevi, digital clubbing, cyanosis, dyspnea | Clinical examination and pulse-oximetry | Contrast enhanced echocardiography and blood gas analysis | Resolution after LT in most cases |
| Portopulmonary hypertension | 4% | - | Asymptomatic, dyspnea, syncope | Echocardiography | Right heart catheterization | Improved survival rates when associated to vasodilation therapy |
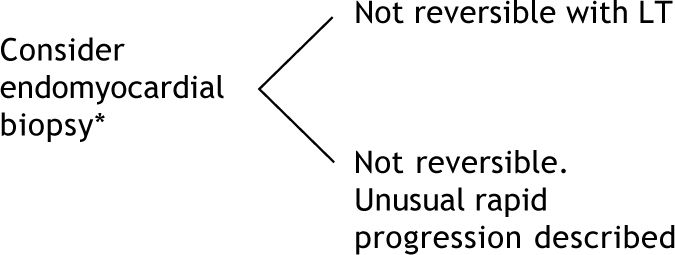
LT: Liver transplantation. Endomyocardial biopsy for demonstration of cardiac tissue infiltration should be only required if less invasive methods fail to enable diagnosis of amyloidosis.69.
Since cardiovascular and respiratory complications are a common cause of complications after liver transplantation, the emphasis will be placed on cardio- respiratory evaluation of the LT candidate (Table 2).
Key messages of the pre operative the cardiopulmonary assessment in the liver transplant candidate.
| 1. | The inherent risk of LT surgery and the shortage of donors mandate the selection of patients who are likely to obtain a significant survival benefit from transplantation. |
| 2. | Preexisting cardiopulmonary disease may have a significant impact on LT outcome. |
| 3. | Preoperative assessment should focus on detecting the more common cardiopulmonary disorders found in patients with end-stage liver disease. |
| 4. | Once the diagnosis has been established, further evaluations are usually needed to determine how a specific cardiac or respiratory disease affects both perioperative risk and the long-term expectancy after LT. |
LT: Liver transplant.
The recent interest in cardiac evaluation stems from two facts. In the former, LT recipients today are older and present more comorbidities than 30 years ago.4 Although older studies suggested that patients with ESLD were somehow protected from coronary artery disease (CAD), recent evidence indicates that the prevalence of CAD in those patients is at least similar to, if not greater than other surgical candidates.2,5,6 Moreover, some cardiovascular risk factors such as hypertension, diabetes, hyperlipide-mia or obesity may be more prevalent after LT due to immunosuppressive regimes.7,8 Evidence is also accumulating suggesting there is a cardiomyopa-thy specific to cirrhosis, regardless of its etiology, possibly related to severe cardiac events in this po-pulation.9 In the latter, the hemodynamic stress associated to LT can exacerbate an underlying cardiac pathology. Cardiac events occur in 25 to 70% of patients during and after LT affecting the graft and patient prognosis.6,10,11 When recurrent disease and de novo malignancies are excluded, cardiovascular disease represents the most common cause of death in long-term LT survivors.12,13 Hence, the cardiac pe-rioperative risk assessment of these patients is an increasingly important clinical requirement before transplantation surgery and will likely have a strong impact on postoperative outcomes.
The cardiac evaluation of potential candidates for LT is complex. CAD, hyperdynamic circulatory state and cirrhotic cardiomyopathy are often present candidates. Other less common conditions such as valvular heart disease, restrictive cardiomyopathy or arrhythmia related to familial amyloid polyneuropa-thy also deserve some attention.
Coronary artery diseaseLT can induce, compared with other transplantation surgeries, marked perioperative hemodynamic changes that can adversely affect outcomes in patients with CAD. During the perioperative period a significant increase in metabolic and oxygen myo-cardial tissue demands occurs. Coronary blood flow may be insufficient to meet the oxygen requirements in patients with a significant coronary occlusion. It is known that the presence of CAD has a strong impact on mortality and morbidity in ESLD patients undergoing LT.2,6 In the late nineties, Plotkin, et al., in a very cited retrospective study including 32 patients with CAD that underwent LT, reported a 3-yr mortality rate up to 50% from cardiac complications and a 80% of morbidity among survivors.5 This was substantially greater than the 1-year mortality rate (10%) for all LT recipients or the mortality rate from cardiac complications from other study populations. Zoghbi, et al. pointed out that late cardiovascular related mortality is often seen in patients with CAD or known risk factors with a reported mortality of 16 to 22% within 5 years of LT.14 Interestingly, Diedrich, et al.,15 in a more recent study reported a 3-yr mortality rate of 26% and morbidity of 38% for a group of patients with known CAD that underwent LT. Although these numbers have improved compared to older studies likely because of improvements in selection and management of CAD as well as in LT perioperative management, they continue to be higher than patients with no CAD.
The exact prevalence of angiographically proven critical (obstructive) coronary artery stenosis (> 50% luminal narrowing) in LT candidates is unknown. Data on the prevalence of CAD in patients with cirrhosis vary greatly (from 2.5 to 27 %).5,16–18 The discrepancy could be related to different demographic characteristics of the studied populations, different prevalence of risk factors and the screening studies performed. The optimal screening strategy for LT candidates is a topic of substantial debate because of conflicting data available for noninvasive stress testing and different algorithms used in several centers. Preoperative workup must include a careful cardiovascular history to detect cardiovascular symptoms and risk factors and to establish the suspicion of cardiac disease estimating the exercise capacity.19–21 However, the limited physical status and poor functional capacity characteristic of patients with ESLD presents a particular challenge because those patients may not experience common cardiac symptoms that are usually provoked by exercise such as chest pain or shortness of breath. Consequently, nearly all CAD patients in the setting of LT are asymptomatic and it is therefore necessary to detect silent CAD. Patients with clear symptoms suggestive of CAD undergo a cardiac stress test or directly a selective coronary arteriography in some institutions (Table 3).
Key messages of coronary artery disease in liver transplant candidates.
| 1. | Careful cardiovascular history to detect cardiovascular symptoms and risk factors as well as estimate of the exercise capacity is required to establish the diagnosis of CAD in LT candidates. |
| 2. | Patients with poor (MET level < 4) or unknown functional capacity should be undergo noninvasive stress testing according to the number of the pre-defined clinical risk factors. |
| 3. | Whereas a positive stress test mandates a selective coronary arteriography to confirm CAD, negative one defines a low-risk patient. |
| 4. | Tests measuring global physical function help determine both long-term prognosis and perioperative risk. |
| 5. | CAD is one of the most prevalent cardiac diseases in ESDL patients. Given the impairment of metabolic profile after LT, post-transplant care should include assessment for risk factors. |
CAD: Coronary artery disease. MET: Metabolic equivalent of task. ESDL: End-stage liver disease. LT: Liver transplant.
Following the recommendations from the American Heart Association/American College of Cardiology for perioperative evaluation for non cardiac surgery,21 the use of noninvasive stress testing as a diagnostic tool for risk stratification in a set of selected patients should be the first step. In the general population, patients with poor (MET level < 4) or unknown functional capacity should be undergo further cardiac evaluation according to the number of defined clinical risk factors.20,21 However, previous studies have failed to identify a consistent risk factor profile in ESLD patients and there is no optimal risk stratification strategy.22 Traditional cardiovascular risk factors like age of over fifty, male sex and diabetes are very frequent in LT candidates and their presence is not always associated with a good predictive value.23,24 In a retrospective cohort analysis of LT candidates aged over 45 years without known CAD, coronary angiography showed a high prevalence (26%) of moderate to severe CAD.18 This study also found that patients with more than 2 cardiovascular risk factors (other than age); men, history of hypertension and/or diabetes had a significant positive trend for the presence of CAD. A diagnosis of non-alcoholic steatohepatitis (NASH) independently increases the risk of CAD.25
Stress testing remains the cornerstone of nonin-vasive cardiac evaluation because it can provoke is-chemic signs and symptoms in patients with occult and hemodynamically significant disease. A positive stress test mandates a coronary angiography in LT candidates. Given the poor physical status in this population, a drug stress test is preferable to graded exercise testing although the results vary. Dobuta-mine stress echocardiography (DSE) and myocardial perfusion scintigraphy (MPS) with gated single-photon emission computed tomography (SPECT) are the most used screening tools to detect CAD in LT candidates. Whereas abnormalities on those tests correlates well with the presence of CAD in the general population,21 unfortunately, the reported sensitivities and specificities in patients with ESLD are more variable ranging from 12 to 100% and 57 to 100%, respectively.26 Direct inotropic stimulation with dobutamine (B-agonist) seems to be better than adenosine or dipyridamol for inducing ischemia with either technique. The state of vasodilatation present in liver failure would render the vasodilator provocation with adenosine or dipyridamol insufficient to induce the necessary increase in coronary blood flow. The theoretical advantage of dobutamine is limited in patients who fail to reach the target heart rate and peak double product (heart rate multiplied by blood pressure) even using atropine because many are treated with betablockers27 or present what is called chronotropic incompetence.28
MPS had a sensitivity of 37% and specificity of 63% to detect significant (> 70%) coronary lesions in a series of 94 patients who underwent both imaging and coronary arteriography.29 Attenuation artifacts due to ascites, which can mimic ischemia or an infarct, may explain the low specificity.30 In a previous study, a subset analysis compared coronary angiography with DSE, and found that DSE was 75% sensitive and 57% specific in detecting CAD.17 DSE is the preferred test in most of LT centers not only because it seems to be more specific than MPS but also provides additional information regarding overall left ventricular function and valvular disease. In addition, it is noteworthy to point out that be-cause of its high reliability in terms of negative prediction; a normal cardiac stress test usually allows to rule out CAD identifying a very low risk group for cardiac events.
Interestingly, despite the imperfect sensitivity and specificity for detecting angiographically defined CAD, both DSE and MPS have prognostic value for cardiac events and mortality in the ESLD population.14,28,31,32
Recently, the development of cardiac computed tomography for the detection and quantification of coronary artery calcification has improved cardiovascular risk prediction in general population compared with the Framingham score in CAD asymptomatic patients. The coronary artery calcification score is an established and robust minimally invasive clinical method for cardiovascular risk as-sessment.33 McAvoy, et al., in a recent study demonstrated a strong relationship between coronary artery calcium score and a number of known cardiovascular risk factors in the setting of LT.34 Although the usefulness of using calcium score in predicting cardiovascular events after LT requires prospective evaluation, it seems promising based on the results in other populations.
Several biomarkers of myocardial injury, such as cardiac troponin and N-terminal pro-B-type natriu-retic peptide, have been proposed as tools in the cardiac risk stratification of patients candidates for kidney transplantation.35 The role of these markers has not been validated in LT candidates although it appears that high levels of brain natriuretic peptide may partially reflect ventricular stress because of cardiac dysfunction36 and, pre-transplant elevated troponin levels, diabetes, and a history of cardiovascular disease, alone or in combination, have been strongly associated with the occurrence of post-transplant cardiovascular events.37 Hence, coronary artery calcium score and/or biomarkers might provide an additive benefit to the assessment of cardiovascular risk profile in candidates for LT, improving the specificity of cardiac stress testing. In the near future, emerging anatomical imaging for identification of atherosclerosis and functional imaging for assessment of myocardial perfusion techniques may also be complementary and improve the risk stratification of CAD38 in ESLD patients.
Once significant obstructive CAD has been identified, however, it is entirely unclear how best to manage these patients. LT should be delayed to allow the institution and titration of appropriate medical therapy, such as statin or B-blocker therapy,39–41 and/or even percutaneous coronary intervention. However, it is not clear whether surgical revascularization, often encouraged in these patients based on small studies suggesting benefit,42 influences outcome relative to transplantation status. In the general population, prophylactic revasculari-zation in the absence of significant ischemia or on-going symptoms seems does not seem not superior to conventional medical therapy.20 Further studies in this specific population undergoing LT are needed before definite recommendations can be made.
Cirrhotic cardiomyopathyThis condition was coined to define a chronic cardiac dysfunction which is evident under stress conditions and affects an indeterminate number of patients with ESLD regardless of etiology of liver disease.9,43,44 It was initially mistakenly attributed to latent alcoholic cardiomyopathy that is usually a dilated cardiomyopathy characterized by increased ventricular volumes and a decreased ejection fraction. Chronic high cardiac output characteristic of ESLD patients has been invoked as the cause of the constellation of cardiovascular abnormalities in some patients with cirrhosis. Although the clinical presentation can be variable, all patients have common features such as baseline increase in cardiac output, attenuated systolic contractile response to stress, diastolic dysfunction and electrophysiological abnormalities, including chronotropic incompetence and prolonged QT interval in the absence of other known cardiac disease.9,44,45 The impaired systolic response to stress and diastolic dysfunction are underlying causes in the increased incidence of pulmonary edema and congestive heart failure following procedures such as transjugular intrahepatic por-tosystemic shunt that abruptly increase blood flow to the heart.46,47 Recently, Ripoll, et al. demonstrated in a series of 209 patients undergoing LT a high in-cidence of an abnormal cardiac response after reper-fusion of the liver graft. There was a significant decrease in left ventricular stroke work index despite a rise in filling pressures (as would be expected according to the Frank Starling mechanism).48 The abnormal response influenced the early postoperative period. This systolic dysfunction may explain a high incidence (18%) of pulmonary edema observed in the early postoperative period.49 The presence of chronotropic incompetence as a single finding has also been associated with a higher rate of periopera-tive complications.28 Although the relevance of cirr-hotic cardiomyopathy on LT outcomes is unknown, intuitively, the severity of this syndrome may explain the high incidence of cardiac complications during and after LT.
To date there is no single diagnostic test to identify this entity and most patients with cirrhotic car-diomyopathy are diagnosed once they develop signs or symptoms of heart failure under conditions of stress. Decreased distensibility of the heart is the hallmark of diastolic dysfunction. Pulsed wave Do-ppler analysis of the mitral valve inflow by echocar-diography is used to determine E/A ratio (early to late atrial phases of ventricular filling). A fall in the E/A ratio usually reflects a fall in diastolic compliance (diastolic dysfunction).45 Diastolic dysfunction usually precedes systolic dysfunction that can be assessed by left ventricular ejection fraction mea-surement.50 It has been suggested that response to volume overload or to peripheral vasoconstrictors could be helpful to detect early forms. Brain natriu-retic peptide, a marker of ventricular stretch, and troponin I are often increased in cirrhotic cardiom-yopathy.36,51 Thus, in the absence of strict criteria, cirrhotic cardiomyopathy is diagnosed if there is evidence of either systolic or diastolic dysfunction, together with supporting criteria such as electro-physiological abnormalities or abnormal serum markers. Specific recommendations for listing are not available. In the general population, a decreased ejection fraction (< 40%) in echocardiography is associated with decreased overall survival postoperati-vely and with increased incidence of congestive heart failure.52 There is some evidence that LT can completely reverse the changes of cirrhotic cardiomyopa-thy. Patients who survive the initial post-transplant period have a complete reversal of both diastolic and systolic dysfunction within a mean time of 9 mon-ths.53 No specific therapy can be recommended for this condition, and management of patients with ci-rrhotic cardiomyopathy should be focused on pre-venting left ventricular heart failure and include conventional therapy for lung stasis and diuretics.
Valvular heart diseaseThe literature on structural heart disease and LT is scarce. The incidence of valvular disease in LT candidates is unknown and commonly discovered during the preoperative assessment either clinically or on the screening echocardiography in most of cases. The presence of valvular heart disease is also considered to be a risk factor for perioperative cardiac complications in patients undergoing noncardiac surgery. Current recommendations for preoperative evaluation of patients with valvular heart disease should include an assessment of the severity of the valvular disease (valve area and degree of obstruction/regurgitation), the degree of impaired myocardial contractility and the determination of the presence of congestive heart failure.54 The preservation of left ventricular contractility and exercise response seems to be crucial in the decision to give priority for LT.55 The severity of valve dysfunction has been shown highly predictive of perioperative complications.56 The perioperative risk for mild to moderate valvular heart disease without impairment of myocardial function could be acceptable based on the results in the general population.21 However, severe forms are more challenging. In the general population, elective noncardiac surgery is usually postponed or cancelled in patients with clinical or echocardiographic criteria for valve replacement. The mortality risk of noncardiac surgery in those patients who refuse cardiac surgery or who are otherwise not candidates for aortic valve replacement, is approximately 10% but data in LT recipients are lacking. Combined cardiac and LT surgery57,58 and two-stage surgical procedure (cardiac surgery preceding LT)59 has been reported although complex and serious morbidity can occur. In absence of more clinical evidence, decisions should be taken based on the hemodynamic alterations that accompany dysfunction of the cardiac valves as well as the alterations caused by this unique expected surgical procedure.
Cardiac disease in familial amyloid polyneuropathy patientsFamilial amyloid polyneuropathy (FAP) is an inherited autosomal-dominant disease due to mutations of transthyretin (TTR) gene. These abnormal proteins, predominantly produced in the liver, lead to extracellular deposition of amyloid in several tis-sues. LT has been widely accepted as the ultimate curative treatment in order to prevent the progression of deposition of amyloid.60 Cardiac involvement is a major concern in patients with FAP candidates for LT since cardiac complications are prognostic factors for mortality and morbidity after LT.61 The most prevalent FAP among the LT candidates, the Portuguese variant (Val30Met mutation), is commonly associated with cardiac autonomic dysfunction and rhythmic disturbances due to amyloid infiltration of conduction system.62,63 Restrictive cardiomyopathy, reflected by a thickened left ventricular wall and high pulmonary pressure, may be present although it occurs more frequently in non-Val30Met variants64 and it appears to predominantly affect older patients of male gender.63 A recent study confirms that the development of conduction disturbances continues after LT.65 Likewise, a significant progression, irrespective of preoperative signs of cardiomyopathy of septal and left ventricular posterior wall thickness has been observed after LT indi-cating a progression of the amyloid heart disease even for the Portuguese variant.64,66,67 It is mandatory to perform a reliable analysis for arrhythmias in FAP patients by 24-h Holter-ECG recordings.63,68 Echocardiography offers a noninvasive diagnostic approach for monitoring progression of the disea-se.69 Increased atrial septal wall thickening and granular sparkling of the myocardium are highly specific for differentiating cardiac amyloidosis from other causes of left ventricular hypertrophy.69 Right-sided heart catheterization will show a hemody-namic profile indistinguishable from other causes of restrictive cardiomyopathy. The indication for pacemaker insertion is different for FAP patients vs. other patients with arrhythmia.65 This is performed routinely in some institutions in all FAP candidates to prevent fatal cardiac arrhythmias. If extensive cardiac infiltration is present; combined heart-liver transplantation should be considered.70,71 However, experience with combined heart-liver transplantation in FAP patients is limited.
Respiratory EvaluationPre-existing pulmonary disease also has a significant impact on perioperative risk. In general, greater degrees of preoperative pulmonary impairment are associated with more marked intraoperative alterations in respiratory function and a higher rate of postoperative complications that often limit survi-val.72 In the LT context, it is critical to establish not only the diagnosis but also the prognosis of the potential pulmonary coexisting disease. This section discusses the most common pulmonary problems observed in ESDL patients that may impact recipient selection.
Chronic obstructive pulmonary diseaseChronic obstructive pulmonary disease (COPD) is not a complication of liver cirrhosis, however is common in LT candidates.73 In one survey of more than 200 LT recipients, 60% reported a lifetime history of smoking.74 It is estimated that 18% of LT candidates have COPD.73 Little is known about the impact of COPD in LT outcome. Interestingly, in a recent study, COPD did not affect short-term survival, however the exclusion of severe COPD for LT could result in a selection bias.73 Although there are no consistent data, it seems obvious that COPD patients may have diminished long-term survival after LT.75 In addition, it is worth to underline that most of the liver recipients resume smoking which may increase the risk of de novo malignancy.76
The forced expiratory volume in one second (FEV1) is often used to grade the severity of COPD.77 However, the BODE index, a multidimensional grading system, seems to be better than the simple pulmonary function testing at predicting the risk of death from any cause and from respiratory causes among patients with COPD.78 BODE index includes other risk factors, such as the presence of hypoxemia, short distance walked in the 6 min walk-test, severe dyspnea, or low body-mass index which have been associated with an increased risk of death. Therefore, a more accurate respiratory evaluation including the 6 min walk test should be mandatory at least in those LT candidates with moderate to severe COPD.
Hepatic hydrothoraxPleural effusions are uncommon in patients with liver disease. They can occur in about 5–12% of pa-tients.79 Effusions are usually defined as hepatic hydrothorax and they are mostly right-sided and occur in absence of coexisting pulmonary or cardiac di-sease.80 This transudative fluid accumulation has been related to an anatomical defect in the hemidia-phragm and always is associated to the presence of ascites.81 Depending on its magnitude it may be res-ponsible of dyspnea and hypoxemia due to a restrictive respiratory pattern. However, the presence of preoperative hepatic hydrothorax does not have a significant negative influence on postoperative outcome of LT.82,83 For pre-transplant evaluation, an important consideration is the exclusion of other causes for pleural fluid accumulation, such as infection, thromboembolic disease, or metastatic carcinoma. Its management is similar to ascites although preoperative thoracentesis could be needed when chest pain or serious compromise pulmonary gas exchange are present. In cases of refractory hepatic hydrothorax a tranjugular intrahepatic portosyste-mic shunt may be needed for control of the effu-sion.79
LT provides a dramatic improvement of this con-dition.82
Pulmonary vascular complicationsThe association of pulmonary vascular complications and portal hypertension has been well documented. Two distinct pulmonary vascular disorders can be found in patients with liver disease that are clearly different and considered mutually opposite. On one hand, hepatopulmonary syndrome (HPS) is characterized by pulmonary vascular dilations and abnormal gas exchange,84 with a prevalence of up to 20% in LT candidates.85 On the other, portopulmo-nary hypertension (PPHT) is a process defined by pulmonary hypertension, pathologically indistinct from idiopathic forms, associated with portal hypertension that is less common than HPS (4%).86 Conceivably, the pathophysiology of these entities likely depends on an imbalance between vasoconstrictor and vasodilator substances resulting from liver dysfunction, developing HPS if the vasodilator effect predominates or PPHT if vasoconstriction over-looks.86,87 It is known that such pulmonary vascular liver-induced entities influence survival and candidacy for LT recipients and consequently should be diagnosed and the severity graded prior LT.
Hepatopulmonary syndromeThe HPS consists of the clinical triad of chronic liver disease, abnormal pulmonary gas exchange (alveolar-arterial oxygen gradient > 15 mmHg, with or without hypoxemia), and intrapulmonary vasodila-tion.88 This combination is so unique that it strongly points to the diagnosis of HPS even in the presence of other concomitant cardiopulmonary di-sease.89 There are no signs, symptoms, or physical examination hallmarks for HPS. However, the presence of spider naevi, digital clubbing, cyanosis and hypoxemia (an oxygen saturation < 97% on room air) should raise the suspicion for HPS.88 The presence of those abnormally dilated pulmonary vessels, the key component underlying gas exchange abnormalities, must be demonstrated either by cons-trast enhanced echocardiography or by of techne-tium-99m-labeled macroaggregates albumin (99mTcMAA) scanning of extra-pulmonary uptake that additionally offers quantitative results.90 In addition, thoracic high resolution scan may be performed to rule out underlying chronic pulmonary di-sorders which may affect the prognosis. Angiography is not necessary for the diagnosis of HPS and has been recommended only when hypoxaemia is severe, poorly responsive to 100% oxygen breathing and/or a high suspicion exists (via thoracic scanning) for direct arteriovenous communications amenable to embolization.91 Notably, due to the progressive course of HPS, regular follow-up is recommended, at least once a year, including pulse oximetry and/or arterial blood gas levels if necessary.
There is a remarkable improvement or full resolution after LT as opposed to the disappointing failure demonstrated by several pharmacological at-tempts.92 LT is currently considered the only effective therapy for HPS. However, evidence suggests that HPS patients have an elevated postoperative mortality.93,94 Staging of the severity of HPS, based on the degree of hypoxemia,88 is important because severity influences survival and resolution probabi-lities.94,95 Preoperative oxygen arterial pressure (PaO2) of 50 mmHg or less alone or in combination with a 99mTcMAA shunt fraction of 20% or more are the strongest predictors of postoperative mortality.94 The high mortality of LT in HPS with very severe hypoxemia (PaO2 < 50 mmHg) and the lower probability of its resolution after LT in those cases, mandates an individual analysis before accepting those patients as LT candidates.88 Otherwise, because patients with severe HPS (PaO2 < 60 mmHg) have an extremely poor prognosis without transplantation, severe hypoxemia has become a reason not only for enhanced prioritization for organ allocation but also a primary indication for LT.93,96
Portopulmonary hypertensionUnlike HPS, the PPHT has important hemodyna-mic consequences with minimal gas exchange abnormalities. PPHT is defined as pulmonary arterial hypertension in a patient who has coexisting portal hypertension, and no alternative cause of the pulmo-nary hypertension exists. The gold standard defintion is made with right heart catheterization that shows mean pulmonary artery pressure (mPaP) > 25 mmHg at rest and a pulmonary vascular resistance of greater than 240 dynes.s.cm-5.88 The pulmonary artery occlusion pressure less than 15 mmHg used to be included in the definition criteria but the common component of volume overload and also increased cardiac output may increase the wedge pressure confounding the diagnosis.88,97 Limited data suggest that patients with PPHT have a poor prognosis after LT, particularly in moderate to severe forms.98
Screening for PPHT should be routinely performed in all patients being considered for LT. Symptoms of cirrhosis frequently confound PPHT symptoms which are initially subtle99 and many of patients with PPHT are diagnosed in the operating room100 or during LT evaluation. When right bundle branch block in the electrocardiogram and/or prominent main pulmonary arteries on chest radiographs are found, PPHT should be suspected.101 Doppler echocardiography is a sensitive method of detecting the presence of pulmonary hypertension that additionally excludes other common causes of se-condary pulmonary hypertension.102,103 A definitive diagnosis requires invasive hemodynamic confirmation by right heart catheterization.104
Currently, the presence of PPHT with a mPAP exceeding 35 mmHg represents a relative contraindication for LT, while a value > 45 mmHg is considered an absolute contraindication given the reported associated perioperative mortality (up to 80%).88 A Task Force on pulmonary vascular disease associated with liver disease has recommended do not transplant these patients. However, there is now compelling data supporting the use of pulmonary artery hypertension-specific therapies (prostacyclins, endothelin receptor antagonists or phosphodiestera-se inhibitors) with the aim of improving pulmonary hemodynamics and right heart function to make LT feasible.105 Selected patients treated with vasomodu-lating therapies previous to LT could have better long-term survival than those not undergoing LT.106 Moreover, in most of these patients, PPHT gradually resolves within 4 to 6 months after transplantation without requiring long-term medical therapy.
Acute vasodilation testing appears to have a relevant role in PPHT as it identifies patients able to reach a more favorable hemodynamic situation which can be determinant for their management.107 In view of this reported experience, and although, in some way speculative, aggressive vasodilation therapies should be recommended in LT candidates with moderate PPHT (mPAP > 35 mmHg) while they are in the waiting list, and in all those patients who show severe forms (mPAP ≥ 45mmHg) and need to be re-evaluated after therapy several months of in order to decide if they can be accepted as candidates.
ConclusionCardiopulmonary disorders play an important role on perioperative risk and long-term survival of LT recipients. A careful cardiopulmonary assessment is mandatory to definitely accept a patient for LT. Thus it is of great importance not only of diag-nose but also establish the risk-benefit ratio. Indeed, the challenge remains in knowing the immediate pe-rioperative risk and how this coexisting disease affects the long-term expectancy of life after LT. Therefore we need to consider what risk we are willing to take in patients being considered for LT. Results from recent studies based on a global physical function assessment seem to have a promising role in the final decision. Some parameters derived from the 6-min walk and cardiopulmonary exercise testing which are widely accepted to assess the functional status and perioperative risk of patients with a variety of other medical conditions,108 have also been associated with the perioperative risk of LT.109,110 The definitive answer involves complex issues about organ allocation, experience of the transplant center, and overall outcome.
Abbreviations- •
LT: Liver transplantation.
- •
ESLD: End-stage liver disease.
- •
CAD: Coronary artery disease.
- •
DSE: Dobutamine stress test.
- •
MPS: Myocardial perfusion scintigraphy.
- •
FAP: Familial Amyloid Polyneuropathy.
- •
COPD: Chronic obstructive pulmonary disease.
- •
HPS: Hepatopulmonary syndrome.
- •
PPHT: Portopulmonary hypertension.
- •
99mTcMAA: Technetium-99m-labeled macroa-ggregates albumin.
- •
PaO2: Oxygen arterial pressure.
- •
mPAP: Mean pulmonary artery pressure.