Background. Acute liver failure (ALF) is a rare but potentially life-threatening condition and liver transplantation (LTX) remains frequently the only effective therapy. Nevertheless, some patients recover without LTX but the individual indication for or against LTX remains difficult.
Aim. To evaluate maximal liver function capacity (LiMAx) for predicting the prognosis of ALF.
Material and methods. Clinic data of 12 patients was retrospectively analyzed to compare the different liver function test results with the patients’ clinical outcome. Patients were assessed by the LiMAx test, a non-invasive breath test determining cytochrome P450 1A2 capacity using intravenous 13C-methacetin. Statistical analysis compared patients with spontaneous recovery versus non-recovery (LTX or death).
Results. Twelve patients (6 male, 6 female; 49 [11–72] years) with viral hepatitis (n = 2), toxic liver injury (n = 3), or cryptogenic liver failure (n = 7) were analyzed. Seven patients fully recovered from ALF and were discharged without LTX. Three patients died and two underwent LTX. The King’s College Criteria (KCC) was fulfilled in only one out of five patients without recovery. The LiMAx was 19 ± 19 (16–62) for non-recovery vs. 94 ± 119 (39–378) μg/kg/h for recovery (P = 0.018). In contrast, all biochemical parameters [bilirubin (P = 0.106), creatinine (P = 0.343), AST (P = 0.53), ALT (P = 0.876) and INR (P = 0.876) were statistically indistinct. Also the Model for End-Stage Liver Disease (MELD) score did not show a difference [35 ± 4.3 (29–40) vs. 30 ± 11.5 (6–40); P = 0.27].
Conclusions. Maximal liver function capacity determined by LiMAx test is severely impaired in patients with ALF. The LiMAx test might be effective in predicting the individual prognosis and the need for LTX in ALF.
Acute liver failure (ALF) is a rare but potentially life-threatening condition. It is characterized by progressive destruction of hepatic parenchyma with cell necrosis and/ or apoptosis. The genesis of ALF is diverse and includes drug-induced liver injury (e.g. acetaminophen), viral or autoimmune hepatitis, metabolic dysfunction and vascular genesis and yet unknown reasons.1
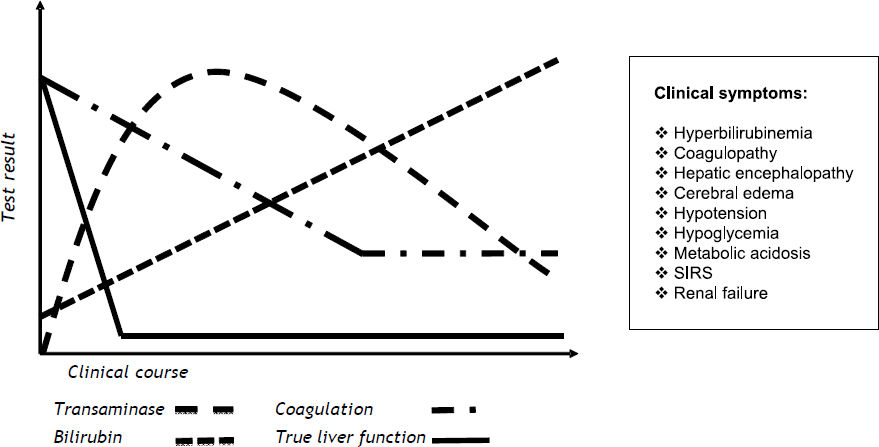
The most widely accepted definition of ALF includes the evidence of coagulopathy, usually an INR > 1.5, and any degree of mental alteration (i.e. hepatic encephalopathy) after exclusion of preexisting cirrhosis and without a disease history of more than 8 weeks.2 In many cases deterioration of liver function leads to multiple organ failure and death. Figure 1 provides an overview of commonly applied biochemical tests and clinical signs for the diagnosis of ALF.
Schematic course of biochemical parameters and clinical symptoms in progressive acute liver failure. Acute liver failure can lead to typical progression of biochemical parameters, such as temporary elevation of transaminases, increasing levels of bilirubin, and coagulopathy. The individual progression of parameters not only depends upon the degree of functional impairment or the severity of liver damage, but also on the particular half-life of parameters in blood. Potentially true liver function is affected at an earlier point in time and decreased faster in comparison to blood parameters. In addition, several clinical signs can lead to the diagnosis of ALF. However, these parameters usually only become apparent in end-stage ALF and indicate a vital risk for the patient.
Although the liver has a relevant regenerative potential, liver transplantation (LTX) remains the single curative therapy in severe cases.3 However, a certain number of patients with ALF fully recover by symptomatic therapy. In these cases LTX would be a relevant waste of resources and could harm patients by severe complications and a long-term application of immunosuppressive agents. Thus the identification of patients with potential recovery is of major clinical importance. In addition, early and accurate assessment of liver injury and function is essential to implement best curative treatment. Specific prognostic factors and scores4–9 were developed to support the crucial process of decision-making, against or in favor of transplantation. The current standard criteria for transplantation are the King’s College Criteria (KCC).10 Although its specificity of 67–86% is somehow acceptable, its reported sensitivity of only 48–68% excludes effective decision making in the assessment of ALF patients.4,11,12 As a consequence the indication for LTX remains a subjective decision on a case-by-case basis by the responsible physicians that includes all patient criteria. Taken together, no reliable diagnostic tools are yet available to predict the individual outcome of ALF. A promising method to overcome this diagnostic gap might be the assessment of the maximal liver function capacity. The LiMAx test enables a realtime in vivo determination of the capacity of the cytochrome P450 1A2 enzymatic system within one hour. Previous clinical trials have demonstrated its sensitivity, specificity and prognostic power in different clinical situations.13–14 The aim of this study was to explore the correlation between LiMAx and the patients’ prognosis in non-acetaminophen-induced ALF in a small pilot study.
Material and MethodsThe retrospective analysis included twelve patients’ hospitalized for ALF that were referred to the Department of Transplantation Surgery to evaluate the indication for LTX. In addition to the routine clinical tests, those patients were assessed by the LiMAx test. The test is currently used in the routine diagnostics of our clinic to assess patients undergoing liver resection and transplantation. The clinic data at the time point of inclusion were analyzed to compare the different liver function test results with the patients’ outcome. In selected patients a second test was performed during hospitalization if requested by the responsible physicians. The LiMAx test was performed as previously described.13 Readouts from prior studies on healthy volunteers showed a normal value > 315 μg/kg/h (311–575).13
The study was approved by the local ethics committee and all patients or their relatives provided written informed consent to undergo the LiMAx test. The study endpoints were either spontaneous recovery with survival, versus non-recovery, defined as LTX or death. The individual therapy and the decision for transplant listing were chosen by the responsible physicians independent from the study protocol. Additional data of clinical findings and biochemical tests were collected from the electronic hospital information system. KCC10 and Model for End-Stage Liver Disease (MELD)15 were calculated for the time point of LiMAx assessment.
Statistical analysisStatistical analysis compared LiMAx readouts, biochemical parameters, KCC and MELD score between recovery and non-recovery. Univariate analysis was performed by Mann-Whitney U test and Fisher’s exact test for independent samples according to the respective data distribution. Data were presented as median with range and SD. To assess the prognostic power of the LiMAx test, receiver-operating characteristics (ROC) were applied. A cut point was set at the maximum sum of sensitivity and specificity. Statistical significance was accepted at P < 0.05 (two-sided). Calculations were performed using IBM SPSS Statistics Version 20.
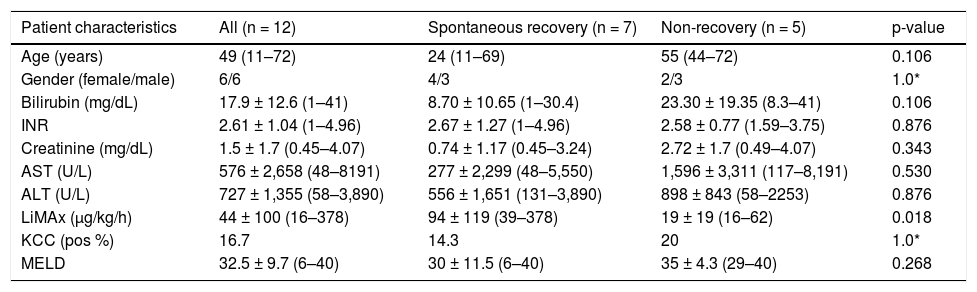
ResultsDetailed patient characteristics are provided in table 1. The LiMAx test was performed 3.5 days (1–12) days after admission to the hospital. The median age of the study group was 49 (11–72) years with equal gender ratio (6 male/6 female). ALF was caused by viral hepatitis (n = 2), toxic liver injury (n = 3), cryptogenic liver failure (n = 7). Seven patients recovered spontaneously and were discharged from the hospital without LTX. In the non-recovery group four patients died including one who had un- dergone LTX. One single patient underwent successful LTX and was discharged in good condition. The causes for ALF among the group with non-recovery were viral hepatitis (n = 2) and cryptogenic liver failure (n = 3). The group analysis indicates differences in biochemical parameters and patient age (Table 1).
Patients’ characteristics.
| Patient characteristics | All (n = 12) | Spontaneous recovery (n = 7) | Non-recovery (n = 5) | p-value |
|---|---|---|---|---|
| Age (years) | 49 (11–72) | 24 (11–69) | 55 (44–72) | 0.106 |
| Gender (female/male) | 6/6 | 4/3 | 2/3 | 1.0* |
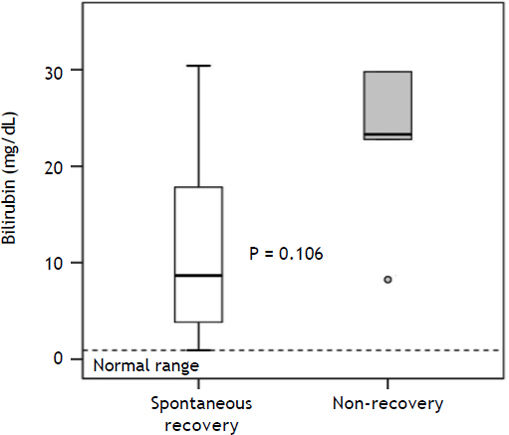
| Bilirubin (mg/dL) | 17.9 ± 12.6 (1–41) | 8.70 ± 10.65 (1–30.4) | 23.30 ± 19.35 (8.3–41) | 0.106 |
| INR | 2.61 ± 1.04 (1–4.96) | 2.67 ± 1.27 (1–4.96) | 2.58 ± 0.77 (1.59–3.75) | 0.876 |
| Creatinine (mg/dL) | 1.5 ± 1.7 (0.45–4.07) | 0.74 ± 1.17 (0.45–3.24) | 2.72 ± 1.7 (0.49–4.07) | 0.343 |
| AST (U/L) | 576 ± 2,658 (48–8191) | 277 ± 2,299 (48–5,550) | 1,596 ± 3,311 (117–8,191) | 0.530 |
| ALT (U/L) | 727 ± 1,355 (58–3,890) | 556 ± 1,651 (131–3,890) | 898 ± 843 (58–2253) | 0.876 |
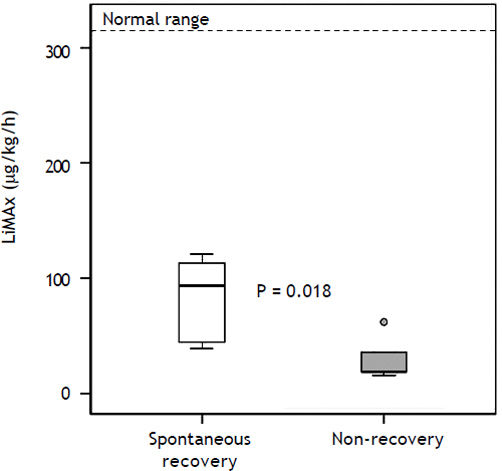
| LiMAx (μg/kg/h) | 44 ± 100 (16–378) | 94 ± 119 (39–378) | 19 ± 19 (16–62) | 0.018 |
| KCC (pos %) | 16.7 | 14.3 | 20 | 1.0* |
| MELD | 32.5 ± 9.7 (6–40) | 30 ± 11.5 (6–40) | 35 ± 4.3 (29–40) | 0.268 |
Median values with interquartile range, analyzed by Mann-Whitney U test.
Median values of bilirubin (P = 0.106) (Figure 2), creatinine (P = 0.343), AST (P = 0.530) and ALT (P = 0.876) were higher in the non-recovery group, whereas INR (P = 0.876) was lower. However, these differences missed statistical significance. The
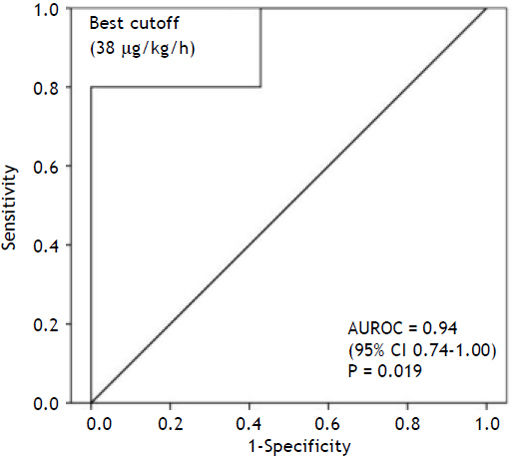
MELD score in the non-recovery group was 35 ± 4.3 (29–40) vs. 30 ± 11.5 (6–40) (P = 0.27). The King’s College Criteria were fulfilled in one out of five patients in the non-recovery group (P = 1.0). In the recovery group six out of seven did not fulfill these criteria. In contrast, LiMAx readouts were 19 ± 19 (16–62) for non-recovery vs. 94 ± 119 (39–378) μg/ kg/h for recovery (P = 0.018) (Figure 3). The ROC analysis indicates an AUROC of 0.94 (95% confidence interval 0.74–1.00, P = 0.019) with a best cut off of 38 μg/kg/h (Figure 4). Thus a LiMAx < 38 μg/kg/h could be predictive for death or need for transplantation with a sensitivity of 80% and specificity of 100% in this small patient cohort.
LiMAx readouts in patients with acute liver failure. Data presented as a box and whisker plot for patient with recovery and patient with non-recovery. Bold lines indicate median, whiskers the min-max range, boxes the interquartile range and circles outliers. The bold text indicates statistical significance (Mann-Whitney U test).
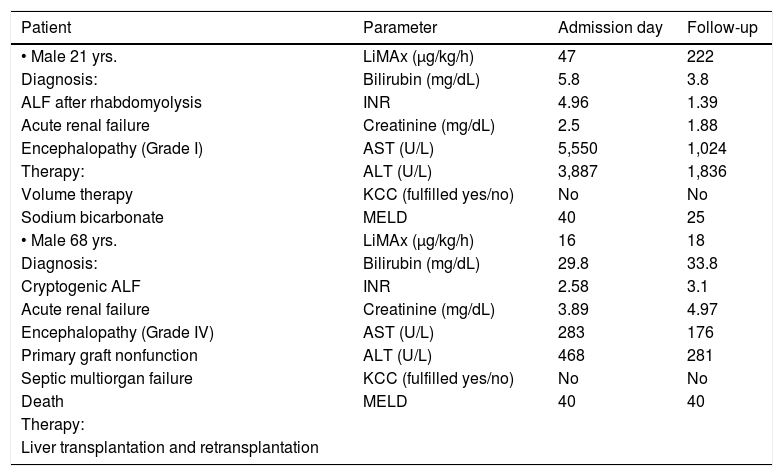
Two selected clinical courses of one patient with spontaneous recovery and another without are expressed in table 2.
Selected case reports of recovery and non-recovery.
| Patient | Parameter | Admission day | Follow-up |
|---|---|---|---|
| • Male 21 yrs. | LiMAx (μg/kg/h) | 47 | 222 |
| Diagnosis: | Bilirubin (mg/dL) | 5.8 | 3.8 |
| ALF after rhabdomyolysis | INR | 4.96 | 1.39 |
| Acute renal failure | Creatinine (mg/dL) | 2.5 | 1.88 |
| Encephalopathy (Grade I) | AST (U/L) | 5,550 | 1,024 |
| Therapy: | ALT (U/L) | 3,887 | 1,836 |
| Volume therapy | KCC (fulfilled yes/no) | No | No |
| Sodium bicarbonate | MELD | 40 | 25 |
| • Male 68 yrs. | LiMAx (μg/kg/h) | 16 | 18 |
| Diagnosis: | Bilirubin (mg/dL) | 29.8 | 33.8 |
| Cryptogenic ALF | INR | 2.58 | 3.1 |
| Acute renal failure | Creatinine (mg/dL) | 3.89 | 4.97 |
| Encephalopathy (Grade IV) | AST (U/L) | 283 | 176 |
| Primary graft nonfunction | ALT (U/L) | 468 | 281 |
| Septic multiorgan failure | KCC (fulfilled yes/no) | No | No |
| Death | MELD | 40 | 40 |
| Therapy: | |||
| Liver transplantation and retransplantation |
INR: international normalized ratio. AST: aspartate-aminotransferase. ALT: alanine-aminotransferase. KCC: King’s College Criteria. MELD: model for end-stage liver disease.
The present pilot study presents for the first time the clinical significance of the maximal liver function capacity in ALF. It is shown that the LiMAx test cannot only determine ALF itself, but can potentially differentiate between those patients with spontaneous recovery and those that require urgent listing for LTX. The lack of diagnostic tests to predict the individual outcome of ALF was the reason to evaluate the potential of the LiMAx test for this indication. The LiMAx test has previously been shown to enable superior accuracy in the assessment of liver function prior to liver surgery13 and after LTX.14,16
The main focus of this study was the comparison of LiMAx test with biochemical testing, the KCC and the MELD score between patients with spontaneous recovery and non-recovery. Although laboratory parameters and MELD differ between these groups, the results failed statistical significance. This is obviously due to the limited size of our study population. Nevertheless, the LiMAx results already achieved statistical significance despite the small sample size. This is of particular importance since a valuable decision for or against transplantation in ALF patients does require diagnostic tests with an individually accuracy for outcome prediction. Most biochemical liver parameters only correlate with non-recovery but cannot predict it validly. Therefore various factors were included into scores in order to improve the diagnostic accuracy.
Today’s most widely utilized tool for the indication of LTX in ALF is the King’s College Criteria.1,3
In literature its accuracy is controversially discussed.12,17 McPhail, et al. investigated in a recent meta-analysis the prognostic power of KCC in patients with non-acetaminophen-induced acute liver failure.12 Their findings indicated a specificity of 80% and a moderate sensitivity 70%. This means the KCC failed to identify 30% of patients requiring LTX. Another meta-analysis comparing different prognostic parameters to detect need for transplantation in patients with acetaminophen-induced ALF provides similar results.17 In the present study only one patient in each group met KCC, so its lack of sensitivity and low negative predictive value are supported by our findings. An additional reported limitation of the score is the rapid clinical deterioration in some patients who fulfilled KCC. Thus, LTX became contraindicated.18
The Model for End-Stage Liver Disease score15 is commonly utilized in predicting 3-month survival of patients with chronic liver disease. It can be used to assess disease severity and it is a widespread-used criterion for organ allocation. A study of Kremers, et al. assumed that a high MELD score in patients with non-acetaminophen induced ALF indicates poor prognosis and therefore listing for LTX is recommended.19 Another study on 109 patients proposed a cut off value of 32 to discriminate between patients with spontaneous recovery and those with non-recovery.20 In the present study, the MELD was > 30 in all patients with severe ALF and did not allow any differentiation between potential recovery and non-recovery. Other studies confirmed that the MELD score cannot provide an adequate positive predictive value (PPV) and has only moderate specificity.21,22 Various studies failed to demonstrate any advantage of its use in patients suffering from ALF.23,24 Taken together, MELD was implemented to detect liver function in patients with chronic liver disease and not ALF, which has a completely different pathophysiology.25
The present study could be criticized for its small study population. Since ALF is a relatively rare condition with heterogeneous etiologies and variable progression, clinical studies on this issue frequently suffer from a relatively small sample size. It remains a challenging issue to perform a controlled prospective study with a homogenous patient population.
Furthermore, the timing of measurements was clinically selected and thus somehow arbitrary. Patients were assessed at the point of time of referral to the transplant center. Therefore the presented sensitivity and specificity of LiMAx for non-recovery can only be seen as preliminary. The ROC analysis was mainly performed to provide a certain cut off value for clinical decisions. Interestingly, the cut off value of 38 μg/kg/h for a negative outcome is slightly lower in comparison to liver transplantation (64 μg/ kg/h)14 and liver resection (74 μg/kg/h).13 Potentially perioperative stress requires a certain magnitude of functional reserve to avoid severe complications, whereas non-surgical patients might tolerate hepatic impairment to a greater extent.
The detailed case reports show the potential application of the LiMAx test in patients with ALF. The definite advantage of the LiMAx test is its sensitivity for short-term changes that cannot be provided by any biochemical test. This has been particularly shown after liver resection, when the common liver function parameters remain unaltered within some hours after surgery, whereas the LiMAx is directly reduced according to the residual liver.13 This phenomenon is evident in the first patient example of this paper. While bilirubin slightly decreased from 5.8 to 3.8 mg/dL the LiMAx increased from 47 to 222 μg/kg/h. Thus the LiMAx enables short-term follow-up assessment of ALF patients. Patients with constantly poor function (approx. 30–50 μg/kg/h) seem to be prone of developing end-stage ALF and should be considered for transplant listing. In contrast, patients with increasing LiMAx results during follow-up are likely to recover spontaneously. Obviously, further studies are necessary to confirm these results and the chosen cut off points.
In conclusion, maximal liver function capacity determined by the LiMAx test is markedly impaired in patients with ALF. The extent of functional impairment might be a very valuable parameter for predicting the individual prognosis and the need for LTX.
Abbreviations- •
ALF: acute liver failure.
- •
ALT: alanine-aminotransferase.
- •
AST: aspartate-aminotransferase.
- •
AUROC: area under the receiver operating characteristic curve.
- •
INR: international normalized ratio.
- •
KCC: King’s College Criteria.
- •
LTX: liver transplantation.
- •
MELD: Model for End-Stage Liver Disease.
- •
PPV: positive predictive value.
- •
ROC: receiver-operating characteristic.
Martin Stockmann is the inventor of the LiMAx test and has capital interest in Humedics, the company marketing the LiMAx test.
Financial SupportNone.