Introduction. Sirolimus has inhibitory effects on epithelial healing and cholangiocyte regeneration. In liver transplantation (LT) patients, these effects may be greatest at the biliary anastomosis. We therefore investigated whether sirolimus use is associated with need for early or emergent repeat therapeutic endoscopic retrograde cholangiography (ERC) in LT patients with anastomotic biliary stricture (ABS).
Material and methods. Medical records of patients who underwent LT from 1998–2009 at Johns Hopkins were reviewed and patients with ABS identified. Primary outcome was early repeat ERC, defined as need for unscheduled (i.e. unplanned) or emergent repeat therapeutic ERC. Univariate and multivariate logistic regression analyses (adjusting for age, sex, LT to ERC time, and stent number) were performed to assess association between sirolimus and early repeat ERC.
Results. 45 patients developed ABS and underwent 156 ERCs total. Early (median 26 days) repeat ERC occurred in 14/56 (25%) and 6/100 (6%) ERCs performed with and without concomitant sirolimus-based immunosuppression, respectively (OR 1.22; 95% CI 1.02–1.45; p = 0.03). In multivariate analysis, sirolimus use was associated with early repeat ERC (OR 1.24; 95% CI 1.04–1.47; p = 0.015); this association remained significant when sirolimus dose was modeled as a continuous variable (OR 1.04 for each mg of sirolimus per day; 95% CI 1.02–1.08; p = 0.038).
Conclusions. Sirolimus-based immunosuppression appears to be associated with a modest but significantly increased, dose-dependent risk of early repeat ERC in LT patients with ABS. Prospective studies are needed to further investigate these findings and determine if sirolimus use or dose should potentially be reconsidered once ABS is diagnosed.
Biliary strictures are a common complication after orthotopic liver transplantation (LT), occurring in up to 17% of brain dead and 50% of donation af- ter cardiac death (DCD) donor liver recipients despite improvements in organ transplantation.1–3 Recent evidence suggests that their incidence has in fact increased in the post-Model for End-Stage Liver Disease (MELD) era.4 In addition to being common, biliary strictures are associated with substantial morbidity and costs, a portion of which stems from frequent and repeated therapeutic endoscopic retrograde cholangiography (ERC).2,5–11 While previous studies have examined the predictors of biliary stricture formation (e.g. DCD donors, hepatic artery thrombosis) and of final endoscopic treatment outcome,2,3,8,9,12–16 the factors predisposing to interval need for untimely (i.e. early) or emergent repeat therapeutic ERC in patients undergoing treatment of post-LT biliary strictures remain unknown.11
One such risk factor may be the immunosuppressive regimen. For example, the mammalian target of rapamycin inhibitor sirolimus is frequently used as an immunosuppressant in LT and is known to have antiproliferative effects on smooth muscle, endothelial, and epithelial cells, including those of the biliary tree (i.e. cholangiocytes).17–21 Indeed, we and others recently identified an independent association between sirolimus use and:
- •
Increased incidence of recurrent biliary obstruction necessitating early or emergent repeat therapeutic ERC22 and
- •
Increased complications of ERC.23
The former association was found post-hoc to be strongest in the subset of patients with anastomotic biliary strictures (ABS), i.e. those occurring at the choledochocholedochostomy (CDCD) due to technical issues, as opposed to nonanastomotic biliary strictures (NABS), which occur elsewhere and through different mechanisms.16
It seems biologically plausible that the adverse biliary effects of sirolimus may be most pronounced at the CDCD, with possible sequelae including impairments in anastomotic healing, ABS remodeling, and bile flow. These sequelae may ultimately translate into adverse outcomes during ERCs needed post-LT. Here we investigated whether sirolimus-based immunosuppression is associated with increased odds of early and emergent repeat therapeutic ERC in patients undergoing endoscopic treatment of post-LT ABS.
Material and MethodsThis study was approved by the institutional review board at Johns Hopkins Hospital/Johns Hopkins University School of Medicine.
PatientsThe electronic medical records of 559 adult patients who underwent LT at Johns Hopkins Hospital between January 1998 and October 2009 were retrospectively reviewed for clinical, laboratory, and radiographic data, and patients with post-LT ABS were identified. Patients who underwent living-donor LT or Roux-en-Y choledochojejunostomy or hepaticojejunostomy or developed both NABS and ABS were excluded. Patients were also excluded if they were diagnosed with hepatic artery thrombosis or if they had ABS that was managed primarily by interventional radiology or surgery.
ABS was defined as a dominant narrowing at the anastomosis with significant impairment of contrast material flow at cholangiography.1 Ductal narrowing without proximal biliary dilatation or contrast flow impairment was not considered sufficient for the diagnosis of ABS. The overall incidence of ABS and NABS (± ABS) at our institution during the study period was 8.8 and 4.5%, respectively.
All patients were initially on a post-LT immunosuppressive regimen consisting of tacrolimus or sirolimus, corticosteroid, and mycophenolate mofetil. The latter two medications were tapered off at approximately 3 months and 1 year after LT, respectively. The selection of tacrolimus vs. sirolimus was based on the presence of renal dysfunction and/or intolerance to calcineurin inhibitors and irrespective of bile duct-related concerns or complications.
Post-LT stricture managementPatients with abnormal liver biochemistries (e.g. total bilirubin) suggestive of biliary obstruction and/ or imaging studies demonstrating dilated intra- or extra-hepatic bile ducts with no alternative diagnosis (e.g. recurrent viral infection, hepatic artery thrombosis, etc.) by clinical, laboratory, imaging, or histological assessment underwent ERC for evaluation.
ERCs were performed with a therapeutic side-viewing endoscope (TJF 160, Olympus America, Inc., Center Valley, PA) with patients in either the prone or supine position by an experienced therapeutic endoscopist. Antibiotics were administered in the peri-procedural period in patients with suspected cholangitis. A Tri-Tome PC with 30 mm cutting wire (Cook, Winston-Salem, NC) preloaded with a 0.035 inch, 450 cm guidewire (Jagwire, Boston Scientific, Natick, MA) was used to achieve biliary cannulation. Biliary sphincterotomy, balloon dilation, and number of stents inserted were at the discretion of the endoscopist. The type of stent used was the Cotton-Huibregtse biliary stent (Cook, Winston-Salem, NC), 90% of which were 10 French (remainder 7 or 8.5 French).
Stent exchange was routinely performed at approximately 3 month intervals or sooner if clinically indicated by signs or symptoms of recurrent biliary obstruction. Stent therapy was continued for up to one year or until the cholangiographic appearance of the strictures was improved over baseline with accompanying resolution of impaired contrast flow, proximal ductal dilation, and/or biochemical liver test abnormalities.
Outcome and variablesThe outcome of this study was occurrence of early or emergent repeat therapeutic ERC (hereinafter “early repeat ERC”). This outcome was defined as an unscheduled (i.e. unplanned) or emergent ERC accompanied by any one of the following signs suggestive of biliary obstruction and/or stent occlusion in the absence of an alternative cause (e.g. recurrent viral hepatitis, hepatic artery thrombosis, pneumonia): temperature > 38.0 Celsius, serum hyperbilirubinemia ≥ 2x upper limit of normal (1.2 mg/dL) or ≥ 1.5x the value prior to stent insertion if it was already above normal, clinical cholangitis, or new (i.e. recurrent) bile duct dilatation on ultrasonographic imaging or computed tomography proximal to the ABS.11 The time interval to repeat ERC was compared between early and non-early repeat ERCs to confirm that it was significantly shorter among the former.
Use of sirolimus-based immunosuppression at the time of ERC was evaluated as the principal predictor (i.e. independent variable) of early repeat ERC. In addition, the following patient-, LT-, and ERC-related variables were assessed as co-predictors: recipient age at LT, sex, race, LT indication, donor type (brain death versus cardiac death), donor age, donor-recipient sex mismatch, cold ischemia time, T-tube (or enteral stent) placement at the time of LT, time from LT to ABS diagnosis, late ABS (i.e. diagnosed > 6 months post-LT), cohort/time period (i.e. patients diagnosed with ABS before as compared to after the median ABS diagnosis date), time from LT to each ERC, and number of plastic stents inserted at each ERC.
Data analysisIn the primary analysis, predictors of early repeat ERC were evaluated with logistic regression analyses on a by-procedure basis (i.e. at the level of the ERC). ERC level analysis was chosen given sirolimus use and dose are subject to change from one ERC to the next, thus some patients:
- •
Were not on sirolimus throughout their endoscopic treatment period or
- •
Did not have a fixed sirolimus dose.
Patient level clustering with longitudinal data analysis was taken into account (adjusting for patients with > 1 ERC) to assess the association between early repeat ERC and sirolimus use in univariate and multivariate analyses. Variables adjusted for in multivariate analyses were age at ERC, time from LT to each ERC, and number of stents inserted at each ERC, given their known impact on outcomes of endoscopic treatment of ABS.1,12,13,24
To account for potential confounding by patientand LT-related variables, a separate, patient-level analysis was also performed. Fisher’s exact test and Mann-Whitney test were used for this analysis, from which variables with p < 0.10 were included in the aforementioned multivariate model.
Tests of significance were 2-tailed, with an alpha level of 0.05. Statistical analysis was performed using STATA 11.0 (StataCorp LP, College Station, Texas).
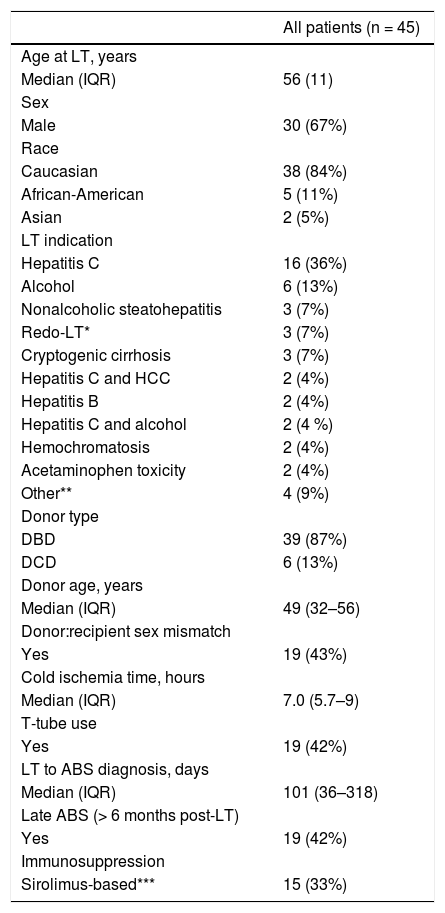
ResultsOf the 45 patients with ABS included in the study, 30 (67%) were male, and the median age at LT was 56 years (range 21–69). Thirty-nine of these 45 (87%) were donation after brain-death (DBD) and 6 (13%) were DCD. The most common indication for LT was chronic hepatitis C infection (36%). Fifteen patients (33%) were on sirolimus-based immunosuppression and had a median sirolimus dose of 2.5 mg/day (IQR 1.5–4, range 0.5–6). Additional characteristics of the 45 study patients are provided in table 1.
Characteristics of patients who underwent LT from 1998–2009 and developed ABS.
| All patients (n = 45) | |
|---|---|
| Age at LT, years | |
| Median (IQR) | 56 (11) |
| Sex | |
| Male | 30 (67%) |
| Race | |
| Caucasian | 38 (84%) |
| African-American | 5 (11%) |
| Asian | 2 (5%) |
| LT indication | |
| Hepatitis C | 16 (36%) |
| Alcohol | 6 (13%) |
| Nonalcoholic steatohepatitis | 3 (7%) |
| Redo-LT* | 3 (7%) |
| Cryptogenic cirrhosis | 3 (7%) |
| Hepatitis C and HCC | 2 (4%) |
| Hepatitis B | 2 (4%) |
| Hepatitis C and alcohol | 2 (4 %) |
| Hemochromatosis | 2 (4%) |
| Acetaminophen toxicity | 2 (4%) |
| Other** | 4 (9%) |
| Donor type | |
| DBD | 39 (87%) |
| DCD | 6 (13%) |
| Donor age, years | |
| Median (IQR) | 49 (32–56) |
| Donor:recipient sex mismatch | |
| Yes | 19 (43%) |
| Cold ischemia time, hours | |
| Median (IQR) | 7.0 (5.7–9) |
| T-tube use | |
| Yes | 19 (42%) |
| LT to ABS diagnosis, days | |
| Median (IQR) | 101 (36–318) |
| Late ABS (> 6 months post-LT) | |
| Yes | 19 (42%) |
| Immunosuppression | |
| Sirolimus-based*** | 15 (33%) |
ABS: anastomotic biliary stricture. DBD: donation after brain death. DCD: donation after cardiac death. HCC: hepatocellular carcinoma. IQR: interquartile range. LT: orthotopic liver transplantation.
A total of 156 ERCs were performed, with a median of 1 plastic biliary stent (range 1–4) inserted at each ERC. Of these 156 ERCs, 56 were performed during sirolimus use (i.e. with patients receiving sirolimus-based immunosuppression). Fourteen out of these 56 (25%) were found to be early repeat ERCs as compared to 6 out of 100 ERCs (6%) performed in patients not receiving sirolimus-based immunosuppression. Notably, the median time to early repeat ERC was 26 days (interquartile range [IQR] 18–51), which was significantly longer (p = 0.002) than all other, i.e. non-early, repeat ERCs (66 days, IQR 49–106). Complications of ERC included 2 cases of pancreatitis and 1 perforation; one of these three complications (pancreatitis) occurred in a patient on sirolimus-based immunosuppression. Biliary stent migration was noted in 4 instances (i.e. 9% of patients, 3% of ERCs); two of these were in patients on sirolimus-based immunosuppression.
In the primary (ERC level) univariate analysis, sirolimus use was significantly associated with early repeat ERC (odds ratio [OR] 1.22; 95% confidence interval [CI] 1.02–1.45; p = 0.03). In the secondary (patient-level) univariate analysis, sirolimus use was again found to be significantly associated with early repeat ERC (p = 0.04), and male sex showed a trend toward an association (p = 0.06) with early repeat ERC (Table 2).
Secondary (patient-level) univariate analysis comparing patient- and LT-related variables between patients who did and did not require early repeat ERC.
| Patients without early repeat ERC (n = 35) | Patients with > 1 early repeat ERC (n = 10) | p-value | |
|---|---|---|---|
| Age at LT, years | |||
| Median (IQR) | 56 (51–61) | 55 (42–60) | 0.49 |
| Sex | |||
| Male | 26 (74%) | 4 (40%) | 0.06 |
| Race | |||
| Caucasian | 29 (83%) | 9 (90%) | 1.0 |
| LT indication | |||
| Hepatitis C* | 17 (49%) | 3 (30%) | 0.47 |
| Donor type | |||
| DCD | 4 (11%) | 2 (20%) | 0.60 |
| Donor age, years | |||
| Median (IQR) | 49 (31–56) | 51 (32–56) | 0.85 |
| Donor:recipient sex mismatch | |||
| Yes | 15 (43%) | 4 (40%) | 1.0 |
| Cold ischemia time, hours | |||
| Median (IQR) | 7 (5.9–9) | 7 (4.8–8.5) | 0.56 |
| T-tube use | |||
| Yes | 15 (43%) | 4 (40%) | 1.0 |
| LT to initial ERC, days | |||
| Median (IQR) | 112 (36–480) | 90 (25–153) | 0.43 |
| Late ABS (> 6 months post-LT) | |||
| Yes | 16 (46%) | 3 (30%) | 0.48 |
| Time period | |||
| First half of cohort** | 20 (57%) | 3 (30%) | 0.17 |
| Sirolimus use | |||
| Yes (any dose) | 9 (26%) | 6 (60%) | 0.04 |
Data reported as median (interquartile range) or number (percent). ABS: anastomotic biliary stricture. DCD: donation after cardiac death. LT: orthotopic liver transplantation.
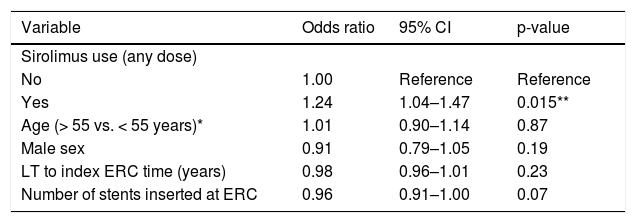
In the multivariate analysis, sirolimus use was significantly associated (OR 1.24; 95% CI 1.04–1.47; p = 0.015) with early repeat ERC (Table 3). To further investigate this association, we modeled sirolimus dose as a continuous variable; we found that the association between sirolimus and early repeat ERC demonstrated a significant, dose-response relationship, with an OR of 1. 04 for each 1 mg increase in the daily sirolimus dose (95% CI 1.02–1.08; p = 0.038).
Multivariate (ERC level) analysis of the association between sirolimus use and early repeat ERC.
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Sirolimus use (any dose) | |||
| No | 1.00 | Reference | Reference |
| Yes | 1.24 | 1.04–1.47 | 0.015** |
| Age (> 55 vs. < 55 years)* | 1.01 | 0.90–1.14 | 0.87 |
| Male sex | 0.91 | 0.79–1.05 | 0.19 |
| LT to index ERC time (years) | 0.98 | 0.96–1.01 | 0.23 |
| Number of stents inserted at ERC | 0.96 | 0.91–1.00 | 0.07 |
CI: confidence interval. ERC: endoscopic retrograde cholangiography. LT: orthotopic liver transplantation.
The development and treatment of biliary strictures continue to be challenging long-term problems in LT patients. This is highlighted by the recent finding of a nearly 3-fold increase in the incidence of ABS in the post-MELD era,4 which is likely in part related to increased utilization of expanded criteria livers. While the factors associated with biliary stricture formation and final endoscopic treatment outcome (i.e. stricture resolution vs. not) have been extensively studied, little is known about which factors may predispose to early interval repeat ERC. In the present study, we found sirolimus-based immunosuppression to be independently and significantly associated with early repeat ERC in patients undergoing endoscopic therapy for post-LT ABS. These results validate, through a larger cohort of patients with ABS, greater number of total ERCs, improved criteria for the primary endpoint, and addition of an important co-predictor (Number of stents inserted at ERC) in our multivariate model, the findings we reported previously in an exploratory study of patients with post-LT biliary strictures.22 It should be noted that although the overall adjusted OR for early repeat ERC in patients taking sirolimus (any dose) was relatively modest, there was a clinically and statistically significant dose-response relationship, with the OR for early repeat ERC increasing by 1.04 for every mg of sirolimus taken per day; therefore, for patients on, for example, 5 mg of sirolimus per day, the estimated adjusted OR for early repeat ERC would be approximately 5.2. The modest overall association in our study (despite this dose-response relationship) was likely related to the somewhat low median sirolimus dose in our cohort.
There may be several reasons for the significant association between sirolimus and early repeat ERC: sirolimus may delay healing of the biliary epithelium and increase or prolong the fragility of the choledochocholedochal anastomosis. Indeed, in rat models of LT, treatment with sirolimus has been shown to be associated with impaired cholangiocyte proliferation,17 and clinical studies have documented delayed epithelial healing in LT patients on sirolimus-based immunosuppression.18–20 Collectively, these effects could increase or protract anastomotic injury and subsequent edema from endoscopic manipulation, to which even a relatively mature anastomosis may susceptible (as supported by the observation that time from LT to ERC was not significantly associated with outcome). In turn, this may lead to decreased cross-sectional area for biliary flow and render the lumen of the biliary stent as the only channel of drainage.
Sirolimus may also potentially predispose to impaired bile drainage through the biliary stent due to:
- •
Changes in bile composition (e.g. more sludging, possibly altered bacterial flora with increased biofilm formation) in the post-operative inflammatory milieu,25 which could be prolonged as a result of impaired cholangiocyte regeneration,17 or
- •
As of yet unknown direct effects of sirolimus on bile composition or flow.
With the use of sirolimus becoming common in the evolving pharmacoscape of immunosuppression as well as the pervasiveness and morbidity of biliary complications, the effects of sirolimus (use, dose, and serum levels) and emerging agents on biliary outcomes, including development of complications (e.g. leaks and strictures) as well as response to treatment, merit additional research.
This study has several limitations. It was retrospective in design, and thus causal relationships cannot be established; therefore, the findings from this study, as with other retrospectively studies, should be validated by prospective studies prior to application to clinical care. In addition, translational studies will likely be required to mechanistically demonstrate whether and how sirolimus may exert deleterious effects on the biliary anastomosis. The sample size was small, albeit larger than most studies of endoscopic therapy of post-LT ABS; using less stringent exclusion criteria may have yielded a larger sample but at the expense of greater sample heterogeneity and confounding. Since the focus of this study was on sirolimus, some but not all demographic, surgical, and endoscopic variables were analyzed. There was no uniform institutional endoscopic treatment protocol in existence during the study period, thus some aspects of endoscopic treatment may have varied over time and between endoscopists; while this may introduce variation that is not accounted for, it may also increase the generalizability of the results. Lastly, although treatment selection bias cannot be completely discounted, as described in the methods, the use of sirolimus-based immunosuppression was unrelated to bile duct-related complications or concerns; furthermore, the existing literature suggests that the incidence of biliary complications (i.e. de novo development) is no different in patients treated with sirolimus (with or without renal insufficiency) compared to those treated with a calcineurin inhibitor.18
In summary, the present study demonstrates that immunosuppression with sirolimus may be modestly but significantly associated with early repeat ERC in LT patients undergoing endoscopic treatment of ABS, particularly at higher daily doses. Given the morbidity and costs of biliary strictures and of early repeat ERCs, these findings suggest a need for larger, prospective clinical and translational studies to better evaluate the impact of sirolimus on the endoscopic management and biology of ABS and to determine if sirolimus use or dose should be reconsidered once ABS is diagnosed.
Abbreviations- •
LT: orthotopic liver transplantation.
- •
DCD: donation after cardiac death.
- •
MELD: Model for End-Stage Liver Disease.
- •
ERC: endoscopic retrograde cholangiography.
- •
ABS: anastomotic biliary strictures.
- •
CDCD: choledochocholedochostomy.
- •
NABS: nonanastomotic biliary strictures.
- •
DBD: donation after brain-death.
- •
OR: odds ratio.
- •
CI: confidence interval.
None.