Management of symptomatic polycystic liver disease (PLD) has remained primarily unchanged since the early 20th century when multiple case reports described management of non-parasitic liver cysts. In 1968, Lin et al. described the fenestration procedure, “aspiration of the cysts, incision, partial excision with or without external drainage, or marsupilization and anastomosis to the gastrointestinal tract”. Further surgical options have included cyst sclerotherapy, laparoscopic cyst aspiration, partial hepatectomy, and orthotopic liver transplant (OLT). Recently there has been discussion of medical management with somatostatin analogs to reduce hepatomegaly in PLD with varying success. There is no current consensus on treatment or standard of care for symptomatic PLD, it is largely up to surgeon preference and ability; however, there has been a movement toward early OLT with Model for End-Stage Liver Disease (MELD) score exception points. This case series reviews two female patients with normal renal and hepatic function with symptomatic PLD treated with transverse hepatectomy. We propose that patients suffering from symptomatic PLD, with retained renal and hepatic function, can be treated with transverse hepatectomy conserving limited donor livers for decompensated patients; moreover, transverse hepatectomy does not disrupt the major suprahepatic vena cava preserving potential surgical access for future OLT.
Polycystic liver disease (PLD) consists of the cystic replacement of healthy liver parenchyma, caused by one of two autosomal dominant forms: isolated hepatic cysts with autosomal dominant polycystic liver disease (ADPLD) or autosomal dominant polycystic kidney disease (ADPKD) with both renal and hepatic cysts [1,2]. The majority of patients with PLD remain asymptomatic, however cystic enlargement can lead to massive hepatomegaly and mass effect within the abdomen causing severe abdominal pain, dyspnea, gastric reflux, early satiety, malnutrition, and chronic fatigue [1,3]. PLD rarely causes derangements in liver function tests, however severe hepatomegaly can lead to portal vein and bile duct compression leading to portal hypertension and hepatic decompensation [1,4]. Moreover, hepatic cysts can rupture or become infected, potentially leading to hypovolemic or septic shock [5,6].
Because liver function is infrequently impaired in PLD, treatment is reserved for patients with symptomatic disease causing reductions and limitations in quality of life and activities of daily living (ADLs) [7]. Medical therapies are infrequently successful in altering patients’ symptoms but are current topics of research, including somatostatin analogs. Somatostatin analogs have been shown to reduce cystic fluid growth by inhibiting cAMP production in cystic cholangiocytes [8]. Multiple randomized control trials have shown reduction in liver volume and minimal reduction in symptoms, however treatment requires continuous use and long-term results are not known [1,9].
Invasive techniques for treatment of PLD revolve around the mechanical reduction of hepatic cyst volume. Less invasive treatment options center around radiologic guided cyst aspiration with and without sclerotherapy agents [10,11]. This treatment is especially useful for large single dominant cysts, however recurrence rates are significantly high approaching 20% [1,10]. Surgical cyst fenestration involves not only aspiration of cystic fluid but unroofing of cysts, and can be done in an open or laparoscopic technique [8]. Cyst fenestration has been extensively described in the literature back into the early 20th century, with multiple techniques to reduce re-accumulation of cystic fluid with marsupialization, anastomosis to gastrointestinal tract, and external drainage [12,13]. However, as with aspiration, recurrence rates are above 20% [8,10].
More definitive surgical options hinge on large resection with or without accompanied cyst fenestration, including anatomic and non-anatomic hepatic resections (including transverse hepatectomy) [3]. A large retrospective single center study, from 1985 to 2014, followed 186 patients who had undergone partial hepatectomy and accompanied cyst fenestration [14]. Median liver volume pre-operatively was 6781 cc and post-operatively was 2502cc, with 61% liver volume reduction [14]. At mean follow-up of 8 years median liver volume was 2519cc, showing continued reduction in liver volume with minimal reoccurrence at prolonged follow-up periods [14].
However, the only curative surgical approach to PLD is orthotopic liver transplantation (OLT) with or without concurrent kidney transplantation [15–17]. Of the near 120,000 people listed for liver transplantation from 2002 to 2015 on the Organ Procurement and Transplantation Network (OPTN), 620 cases were for associated PLD [16]. Although only a minority subset of patients undergo OLT, patients with PLD had a statistically significant longer survival after transplant compared to patients with hepatocellular carcinoma (HCC) and chronic liver failure (CLF) [18]. Increased survival post-transplant and the curative nature of the surgery for patients with PLD leads many centers to push for early transplantation in select cohort of patients [16].
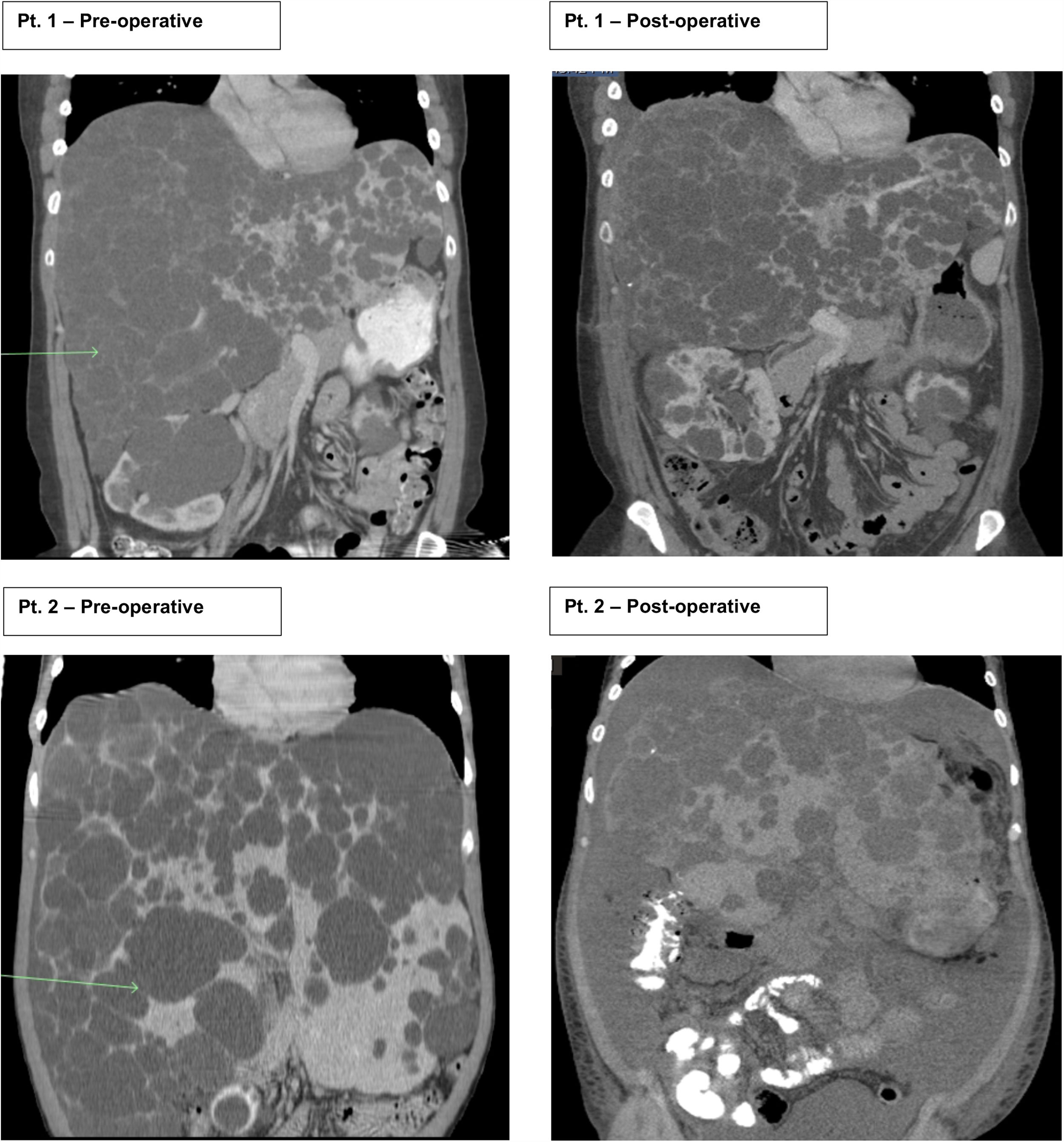
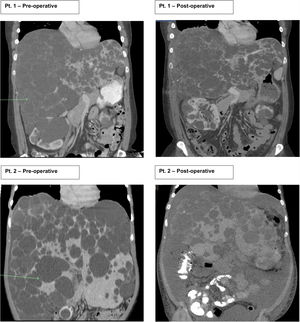
2Case seriesThis is a retrospective case series review performed at one large academic tertiary care center from 2012 to 2018 focusing on transverse hepatectomy for PLD. Two female patients with PLD have been treated with transverse hepatectomy with symptomatic relief since 2014. Both patients had a longstanding diagnosis (greater than ten years) of ADPKD, with strong family history disease penetrance. Patient 1 (43 years old) had an initial incidental diagnosis of PLD due to interval CT scan for monitoring of ADPKD. Patient 2 (39 years old) was diagnosed with PLD after an emergent hospitalization for the discovery of a bleeding liver cyst, which was managed conservatively. Both patients were referred to our medical center for surgical consultation for management of PLD. They complained of severe abdominal distention with limitations to ADLs, shortness of breath with activity, decreased appetite, and early satiety. Patient 2 begun to have evidence of muscle wasting. On physical exam both had massive hepatomegaly with visible and palpable liver cysts protruding below the right costal margin, with palpable liver edges extending into and across to the left lower quadrant and pelvic brim. At presentation both had normal renal and liver function with no laboratory abnormalities. Preoperative CT scans for both patient showed complete replacement of liver parenchyma with cystic lesions, varying from millimeters to greater than multiple centimeters in size (Fig. 1).
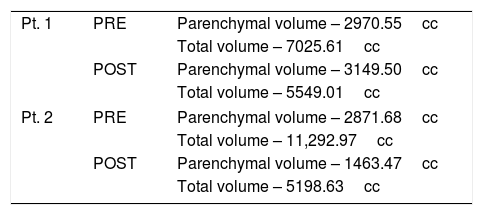
Patients 1 and 2 both underwent nonanatomic transverse hepatectomy, significant unroofing and decortication of remaining liver parenchyma, and cholecystectomy, additionally patient 1 had multiple right renal cysts decorticated. A bilateral subcostal incision was completed, significant adhesions to anterior abdominal wall due to superficial cysts were dissected free to expose segments III, IVB, V, and VI, which were transected using a combination of electrocautery (Argon beam coagulator and Aquamantys bipolar system) and vascular stapling devices. Specific attention was taken to avoid central hepatic veins, deep hepatic parenchymal transection was performed with vascular stapling devices to minimize hepatic vein injury or stenosis with thermal injury from electrocautery. Patient 1 had a pre-operatively liver volume of 7025.61 cc, post-operatively liver volume was 5549.01 cc, with 21% liver volume reduction. Patient 2 had liver volume reduction of 54%. Although patient 1 had a smaller total liver volume reduction, the ratio of parenchymal volume to total volume significantly changed post-operatively, increasing approximately 15% (Table 1).
Volumetric Analysis – CT liver volumetry was measured pre-operatively and post-operatively, by manually segmenting the liver on stack of axial images, creating a segmented 3D liver volume. Quantification was further analyzed comparing parenchymal volume to total volume, indirectly showing cystic capacity of liver.
| Pt. 1 | PRE | Parenchymal volume – 2970.55cc |
| Total volume – 7025.61cc | ||
| POST | Parenchymal volume – 3149.50cc | |
| Total volume – 5549.01cc | ||
| Pt. 2 | PRE | Parenchymal volume – 2871.68cc |
| Total volume – 11,292.97cc | ||
| POST | Parenchymal volume – 1463.47cc | |
| Total volume – 5198.63cc | ||
Blood loss during each operation was less than 1L, both patients were transferred post-operatively to the surgical intensive care unit for close monitoring. Both were discharged home on post-operative day seven and six respectively. Post-discharge course for patient 1 was complicated by right hemi-diaphragm excursion impairment, furthermore a hydropneumothorax was visualized and underwent a thoracentesis with 60cc of transudative fluid removed on post-operative day 75. Symptoms stabilized and improved; however, the patient returned to clinic 5 years post-operatively with recurrence of symptoms of fatigue and increased abdominal girth from hepatomegaly. The patient is being considered for possible OLT and liver function remains within normal limits. Patient 2 had development of ascites post-discharge due to portal hypertension secondary to surgery, requiring large volume paracentesis on post-op day 34 and 76, ascites subsequently controlled medically with diuretics. Diuretics were able to be discontinued, and patient had resolution of ascites. Patient 2 was subsequently found to have thrombocytopenia and evidence of an enlarged spleen, hypothesized to be caused by portal hypertension. At follow up 2 years after surgery, patient 2 still notes complete resolution of symptoms related to abdominal girth and no limitations in ADLs .
3DiscussionThere is little consensus to treatment protocols for patients with symptomatic PLD. Medical treatments, including somatostatin analogs, have limited potential to reverse massive hepatomegaly once patients present with symptoms limiting quality of life (however the use at earlier stages of diseases is still being researched). Surgical interventions, including cyst aspiration, cyst fenestration, and hepatic resection, directly reduce the hepatomegaly that leads to compressive symptoms and discomfort; however, rates of recurrence are high. In this setting patients are presenting for OLT at earlier stages of disease due to complete elimination of disease process and solution of hepatomegaly symptoms [19]. Very few patients qualify for OLT in expedited fashion due to preservation of liver function tests, leading to low biologic Model for End-Stage Liver Disease (MELD) scores. Approximately 40% of patients undergoing OLT for PLD undergo simultaneous liver kidney (SLK) transplantation due to concurrent renal dysfunction, and MELD score is driven by creatinine for these patients [18,20]. However, for patients without concurrent renal dysfunction MELD exception points are needed to drive expedited transplantation. In 2015, the OPTN released the Guidance on MELD PELD Exception Review stating exception points are awarded for limited performance status following resection or fenestration, hepatic decompensation, or concurrent hemodialysis.
Even with MELD exception points only approximately 50% of PLD cases listed for transplant undergo OLT, with over 10% of waitlisted PLD patients dying on the transplant list [16–18]. In this setting more aggressive treatment options need to be undertaken, beyond transverse hepatectomy, discussions need to include split liver transplant (SLT) and living donor liver transplantation (LDLT) to accommodate for patients with PLD and low MELD scores unable to obtain a liver on waitlist [21]. SLT and LDLT are technically difficult in their own right; furthermore, when performed in the setting of PLD with massive hepatomegaly, size mismatch with the graft is a technical issue hard to overcome [21].
Limitations of this study include it being a case series with small sample size, of only two patients, with retrospective data collection thus limiting the external validity when applied to further patient populations. However, the limited prevalence of PLD, moreover the limited prevalence of PLD with preserved liver and renal function, allows insight to be gained from small case series discussing treatment options. Transverse hepatectomy, in select group of patients, provides long term relief of symptoms and potential avoidance of OLT, preventing a patient from requiring lifelong immunosuppressive medication and the multitude of other complications included with organ transplantation. Furthermore, transverse hepatectomy preserves the suprahepatic vena cava and deep hepatic veins preventing significant scarring, a common complaint when aspiration and fenestration procedures fail and OLT is needed. We propose that symptomatic PLD patients with preserved renal and hepatic function explore less invasive mechanical cyst removal, including transverse hepatectomy, to preserve limited donor organ pool and future surgical access for OLT. Moreover, this technique subsequently allows patients who proceed to need OLT MELD exception points under the current OPTN guidelines, since such patients would have failed resection or fenestration.AbbreviationsPLD polycystic liver disease autosomal dominant polycystic liver disease autosomal dominant polycystic kidney disease activities of daily living orthotopic liver transplantation Organ Procurement and Transplantation Network hepatocellular carcinoma chronic liver failure Model for End-Stage Liver Disease simultaneous liver kidney split liver transplant living donor liver transplantation
Thomas W. Smith Jr. – Study concept and design, acquisition of data, drafting of manuscript, critical revision of manuscript. Ari Goldberg – Analysis and interpretation of data, technical imaging interpretation. Amy D. Lu – Study concept and design, critical revision of manuscript, administrative, study supervision.
Conflict of interestNone.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.