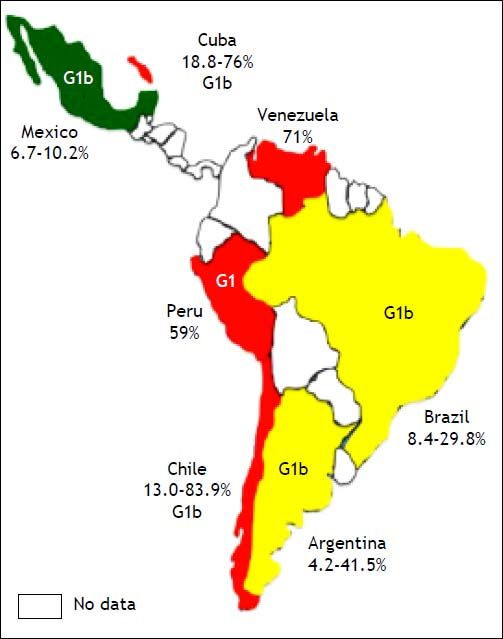
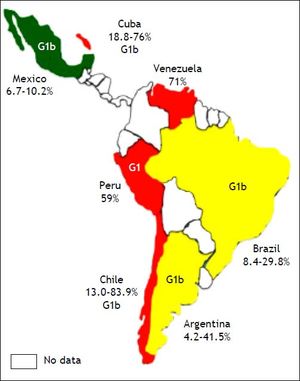
Hepatitis C infection is a worldwide problem. The global prevalence of the hepatitis C virus (HCV) averages 3%. Moreover, its prevalence among patients undergoing haemodialysis (HD) varies worldwide, ranging from as low as 1% to up to 70%. There are few data on its prevalence in developing countries, and even less information is available on HD patients. A literature review revealed that the prevalence of HCV infection among patients undergoing HD in Latin America ranges from 4.2 to 83.9%, with most data stemming from Argentina, Brazil, Mexico, Peru, Chile, Venezuela and Cuba. The most common genotype was genotype 1, and subtype 1b was the most frequent. The risk factors associated with this condition were the duration of the HD treatment and blood transfusion before hepatitis C screening. In addition, HCV RNA detection by polymerase chain reaction is crucial for the diagnosis of HCV infection in HD patients. Trials using combinations of new oral antiviral drugs, such as sofosbuvir and combo (ombitasvir, paritaprevir, ritonavir and dasabuvir), should be the next step in the improvement of care among HD patients with HCV, because these therapeutic agents apparently do not require dose adjustment according to renal function. Finally, information on this subgroup of patients remains unavailable in some countries; therefore, additional studies are needed to determine the prevalence trend of HCV infection in these populations.
The hepatitis C virus (HCV) is a blood-borne pathogen that appears to be endemic in most parts of the world. The World Health Organization (WHO) estimates that the global prevalence of HCV infection averages 3%, which corresponds to about 170 million infected persons worldwide.1 The prevalence of confirmed HCV by first-generation enzyme immunoassay positivity in blood donors ranges from less than 0.1% in Northern Europe to 0.1-0.5% in Western Europe, North America, parts of Central and South America, Australia and a few regions of Africa. An intermediate prevalence (1-5%) has been reported in Brazil, Eastern Europe, the Mediterranean area, the Indian subcontinent and parts of Africa and Asia. The highest prevalence of HCV has been found in Egypt (17-26%).2 In general, between 1990 and 2005, the prevalence of, and the number of people carrying, anti-HCV antibodies increased from 2.3% (95% UI, 2.1-2.5%) to 2.8% (95% UI, 2.6-3.1%) and from > 122 million to > 185 million, respectively.3 Globally, genotype 1 is estimated to account for more HCV cases than any other genotype, at 83.4 million carriers (46.2%), with over one-third of genotype 1 cases stemming from East Asia. HCV genotype 3 is the next most-common genotype and is estimated to account for 54.3 million (30.1%) cases globally, approximately three-quarters of which occur in South Asia. Genotypes 2, 4 and 6 are responsible for the majority of the remaining cases of HCV worldwide, corresponding to an estimated 16.5 million (9.1%), 15.0 million (8.3%) and 9.8 million (5.4%) cases, respectively.4 Petruzziello, et al. described the most common risk factors according to genotype in the Italian population. Dental therapy was the most frequent risk factor for HCV acquisition among individuals with genotype 1 (30.7 vs. the 16.8% observed for genotype 2; P < 0.005), whereas intravenous drug abuse and tattooing were the most prevalent risk factors among patients with genotype 3 (60.0% of the 25 patients with genotype 3 vs. 8.2% of the 376 patients with genotypes 1 or 2; P < 0.0001). Surgery was identified more frequently as a prevalent risk factor in patients with genotype 2 (42.0%) compared with those with genotype 1 (33.8%), albeit without statistical significance.5 The most important source of HCV transmission in developed countries was either parenteral exposure to contaminated blood or illicit use of injectable drugs. The introduction of routine testing of donated blood has decreased the transmission of HCV via blood transfusion; however, illicit use of injectable drugs is currently the main source of HCV infection in most developed countries, accounting for 40% or more of the cases of infection recorded. Other sources, such as nosocomial transmission, are a major problem in developing countries, because of the reuse of contaminated or inadequately sterilized syringes and needles used in medical, paramedical and dental procedures, with an estimated 2.3-4.7 million new infections occurring each year.6
The region corresponding to Latin America has a prevalence of HCV that is among the lowest worldwide, with an overall prevalence estimated at around 1.23%.7 Nevertheless, this prevalence varies from country to country and even between regions of the same country. In 2011, Kershenobich, et al. reported a worldwide prevalence of HCV between 1 and 2.3%, and genotype 1 was the most common genotype. The major risk factors were blood transfusion and use of intravenous drugs, whereas the minor risk factors included nosocomial-related factors, such as surgeries, injections, vial reuse, contaminated tools, and acupuncture/tattooing.8 In 2012, Szabo, et al. estimated that the prevalence of HCV in Latin American ranged from 0.9 to 5.8%, and genotype 1 was also the most frequent genotype.9 It is important to note that one-third of the WHO member countries do not collect prevalence data for viral hepatitis; therefore, its prevalence is underestimated.10
The prevalence of HCV among HD patients varies worldwide, ranging from as low as 1 to up to 70%, and the dialysis-related risk of HCV infection is estimated at 2% per year.11 Overall, the HCV prevalence in patients in HD is below 5% in most countries of Northern Europe, around 10% in most countries of Southern Europe and the United States, and be- tween 10 and 50% and up to 70% in many parts of the developing world, including many Asian, LatinAmerican and North-African countries.12 There are no firm data concerning the distribution of HCV genotype among HD patients. Studies conducted in the Netherlands, France, Morocco, Mexico and Turkey report a predominance of genotype 1b among HD patients. In a study from the United States, subtype 1a was the most frequent in dialysis patients, whereas subtypes 2a and 3a predominated in Italian HD patients. The most common risk factors associated with HCV infection among HD patients were blood transfusion before 1990, the number of blood transfusions, the duration of end-stage renal disease and HD, intravenous drug use and unsafe medical procedures.11,13 The prevalence of HCV among HD patients varies according to HD unit (HU) and is unknown in various stages of chronic kidney disease (CKD) before dialysis or transplantation.2 The aim of this article was to review the HCV prevalence in HD patients in Latin-American countries.
ArgentinaThe incidence rate of end-stage chronic renal disease in Argentina is 152.5 per million persons (pmp), and the prevalence rate of HD is 616.3 per million persons (pmp).14 In 2000, Fernandez, et al. evaluated 108 patients from two different HUs and found that the HCV RNA was present in 19 patients (17.6%).15 Two years later, Valtuille, et al. assessed the variation in the prevalence and long-term incidence of HCV infection in HD patients during a 6year follow-up period. In 1994, 22 out of 53 (41.5%) patients tested positive for anti-HCV antibodies; in 1996, this figure was 18 out of 67 (26.9%) patients; in 1998, it was nine out of 75 (12.0%) patients; and in 2000, it was seven out of 82 (8.5%) patients (P < 0.001). The yearly seroconversion rate was 0.5% during the period 1994-1996 (one out of 98 patients were at risk), 0.5% during the period 1996-1998 (one out of 91 patients were at risk) and 0.4% during the period 1998-2000 (one out of 120 patients were at risk).16 In Argentina, according to the Chronic Dialysis Registry,17 positive HCV-ELISA reactions in individuals entering HD have decreased from 2.0% in 2004 to 1.0% in 2011. The global HCV prevalence in 2011 was reported to be 4.9%. The most common genotype is genotype 1, and subtype 1b is reported most frequently. The risk factors for HCV infection in patients in HD include blood transfusion, and the risk rises with time on HD, being about 38% in HD patients who underwent 16 or more years of treatment18 (Table 1, Figure 1).
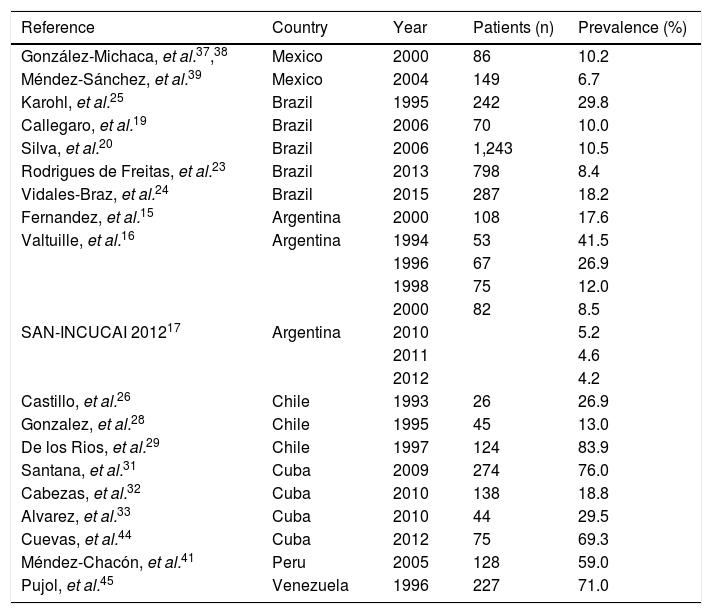
Prevalence of HCV infection in haemodialysis patients in Latin America.
| Reference | Country | Year | Patients (n) | Prevalence (%) |
|---|---|---|---|---|
| González-Michaca, et al.37,38 | Mexico | 2000 | 86 | 10.2 |
| Méndez-Sánchez, et al.39 | Mexico | 2004 | 149 | 6.7 |
| Karohl, et al.25 | Brazil | 1995 | 242 | 29.8 |
| Callegaro, et al.19 | Brazil | 2006 | 70 | 10.0 |
| Silva, et al.20 | Brazil | 2006 | 1,243 | 10.5 |
| Rodrigues de Freitas, et al.23 | Brazil | 2013 | 798 | 8.4 |
| Vidales-Braz, et al.24 | Brazil | 2015 | 287 | 18.2 |
| Fernandez, et al.15 | Argentina | 2000 | 108 | 17.6 |
| Valtuille, et al.16 | Argentina | 1994 | 53 | 41.5 |
| 1996 | 67 | 26.9 | ||
| 1998 | 75 | 12.0 | ||
| 2000 | 82 | 8.5 | ||
| SAN-INCUCAI 201217 | Argentina | 2010 | 5.2 | |
| 2011 | 4.6 | |||
| 2012 | 4.2 | |||
| Castillo, et al.26 | Chile | 1993 | 26 | 26.9 |
| Gonzalez, et al.28 | Chile | 1995 | 45 | 13.0 |
| De los Rios, et al.29 | Chile | 1997 | 124 | 83.9 |
| Santana, et al.31 | Cuba | 2009 | 274 | 76.0 |
| Cabezas, et al.32 | Cuba | 2010 | 138 | 18.8 |
| Alvarez, et al.33 | Cuba | 2010 | 44 | 29.5 |
| Cuevas, et al.44 | Cuba | 2012 | 75 | 69.3 |
| Méndez-Chacón, et al.41 | Peru | 2005 | 128 | 59.0 |
| Pujol, et al.45 | Venezuela | 1996 | 227 | 71.0 |
SAN-INCUCAI 2012, Argentine Chronic Dialysis Registry.
HCV infection has been identified as the major cause of chronic liver disease among patients on chronic HD. The incidence rate of end-stage chronic renal disease in Brazil is 173.7 pmp, and the prevalence of HD is 530.8 pmp.14 In 2006, Callegaro, et al. assessed the prevalence of HCV among 70 patients undergoing HD; seven (10%) patients exhibited anti-HCV reactivity.19 Another study that was published in the same year estimated the prevalence of HCV infection and genotypes among HD patients in Salvador, north-eastern Brazil. The anti-HCV antibody seroprevalence among these HD patients was 10.5% (95% CI, 8.8-12.3%). The HCV RNA was detected in 73.6% of the anti-HCV-positive patients. HCV genotype 1 (77.9%) was the most prevalent, followed by genotypes 3 (10.5%) and 2 (4.6%). Mixed infections of genotypes 1 and 3 were found in 7.0% of the total number of patients.20 In 2008, Oliveira-Penido, et al. evaluated the seroprevalence of HCV in patients who were submitted to HD in the state of Minas Gerais, south-eastern Brazil. Patients from 66 HUs were studied using a validated questionnaire and considering the positive values of anti-HCV (ELISA III) tests performed in 2003. The majority of patients were male (56.2%) and aged between 41 and 60 years. The mean seroprevalence of HCV in the 66 HUs was 13 ± 9.5%. There was a positive correlation between HCV seroprevalence and time on HD in four HUs (P < 0.001).21 Lemos, et al. evaluated the prevalence of, and factors associated with, HCV infection in pre-dialysis patients. A total of 1,041 patients (61% males) with a mean age of 61 ± 15 years were included in the analysis. Forty-one (3.9%) patients were anti-HCV positive and, of these, 39 (95%) presented viremia. Pre-dialysis patients with HCV showed more frequently a history of blood transfusion before 1992 (66.7 vs. 10.3%; P < 0.001) and major surgeries (53.8 vs. 17.1%; P < 0.001), a higher proportion of undetermined aetiology of kidney disease (43.6 vs. 17.1%; P = 0.001) and higher alkaline phosphatase (ALT) levels (1.3 vs. 0.4 x ULN; P < 0.001). A history of blood transfusion before 1992 (OR = 19; P < 0.001), intravenous drug abuse (OR = 69; P = 0.002) and elevated ALT levels (OR = 50; P < 0.001) were variables that were independently associated with chronic HCV infection. The most prevalent HCV genotype was 1b (48.7%), and 56.5% of the patients presented a high HCV viral load.22 Another study reported in 2013 assessed seven dialysis centres located in Belém, Pará, northern Brazil. The authors evaluated 798 patients in HD, and found a prevalence of 8.4% (67) for anti-HCV positivity by ELISA, ranging from 4 to 14% in different centres. Viral RNA was detected in 5.3% (43/798) of the patients; among them, 42 patients also had anti-HCV antibodies. Genotype 1 was the most common genotype; it was detected in 86.1% (37/43) of the patients, followed by genotypes 2 [detected in 11.6% (5/43) of the patients) and 3 (detected in one patient (2.3%)].23 In 2015, Vidales-Braz, et al. reported the highest HCV prevalence among the 318 patients who participated in the study: 55 patients were reactive to anti-HCV antibodies. The prevalence of HCV was 18% (58), and the concordance between the HCV serology and the reverse transcription polymerase chain reaction (RT-PCR) results was 94%. Genotype 1 was the most prevalent (46.7%), within which subtype 1a was the most frequent (74.1%). The length of time that the patient had been undergoing HD was statistically significant risk factor among the HCV-positive patients (P < 0.001), with an average of 101.6 months.24 The results of these studies indicate a significant decrease in anti-HCV prevalence, from the 29.8% detected in a study25 carried out in 1995 to the 10-18% reported in the present study. Several risk factors for HCV infection in patients undergoing HD have been reported for the Brazilian population, such as history of blood transfusion before 1992, intravenous drug abuse and higher ALT levels; however, the most important of these factors remains the duration of HD treatment.
ChileThe incidence rate of end-stage chronic renal stage is 174.9 pmp, and the prevalence of HD is 901.6 pmp.14 In Chile, some studies have determined the prevalence of hepatitis C in HD patients. In 1993, Castillo, et al. evaluated 26 chronic haemodialysed patients and 43 kidney-transplant recipients: seven of the patients undergoing HD had elevated serum transaminase values, all with positivity for anti-HCV antibodies, and 35% of the kidney-transplant recipients exhibited positivity for anti-HCV antibodies.26 In the same year, another study detected anti-HCV antibodies in 30% of patients in an HU, all of whom had been on HD for a longer time than had those with a negative test (53.3 ± 18.8 vs. 37.9 ± 33.5 months, respectively). No differences in the number of transfusions received were observed between patients with or without antibodies.27 In 1995, Gonzalez, et al. determined a prevalence of HCV of 13% (6/45) and a prevalence of the HCV RNA of 6.5% (3/45) among HD patients.28 Moreover, the most common genotype was 1b. In 1997, a prevalence of anti-HCV antibodies of 83.9% was reported, and the only risk factor that was associated with infection was the length of time on an HD program (P = 0.00001). No statistical associations between the level of serum ALP and ALT and anti-HCV-positive tests were found.29
CubaHCV infection was identified as a public health problem in Cuba in the 1990s. Despite universal blood-donor screening, which was achieved in 1995 using the Cuban immunoassay system, ultramicroenzyme-linked immunosorbent assay (UMELISA), for the detection of HCV, the infection is still found in multi-transfused patients. The prevalence of HCV infection in multi-transfused Cuban patients was 51.6% in 2005, and the incidence rate in patients with end-stage chronic renal disease was 99.0 pmp, with a prevalence of HD of 222.6 pmp.14,30 In 2009, the prevalence of HCV infection in HD patients was 76%, and the estimated prevalence determined using viral RNA detection was 55%.31 In 2010, The Dr. Juan Bruni Zayas Alfonso General Hospital in Santiago de Cuba estimated that the prevalence of the anti-HCV antibody among HD patients was 18.8% (26/138).32 In the same year, 44 patients who had received treatment at the HU of the Orlando Pantoja Tamayo Hospital from 2004 to 2009 were assessed. Thirteen of those affected (29.5%) were positive for HCV, as assessed using ELISA. Genotype 1 and subtype 1b were the most common genotypes, and the major risk factors for HCV infection were a longer HD treatment time and multiple transfusions.33 Another study reported a prevalence of HCV of 69.3%, an average age of 45-54 years (21.3%) and male predominance (72.0%). The presence of pre-existing liver disease was the only statistically significant prognostic factor (OR = 4.80; 95% CI, 1.05-21.88), as evaluated via logistic regression analysis. Other factors that were of great interest included exposure to blood (OR = 1.46; 95% CI, 0.48-4.39) and the reuse of dialyzers (OR = 1.38; 95% CI, 0.49-3.92).34
MexicoThe prevalence of HCV infection in Mexico has been estimated to be between 1.2 and 1.5%. Moreover, approximately 1 million individuals are chronically infected with HCV in that country,35 which can be considered to be a significant public health problem. Thus, the design of strategies aimed at a better identification and treatment of a higher percentage of patients with hepatitis C infection is necessary.36 The incidence rate of end-stage chronic renal disease in Mexico is 458.0 pmp, and its prevalence in HD patients is 381.9 pmp.14
In Mexico, few studies have evaluated the prevalence of HCV infection in HD patients. Gonzalez, et al. studied 235 dialysis patients who were classified according to their dialysis modality as follows: 132 patients under continuous ambulatory peritoneal dialysis (CAPD), 17 patients under CAPD with a his- tory of HD, and 86 patients under HD. The presence of hepatitis C was detected in 24 of the 235 patients, yielding a global prevalence of 10.2%. The prevalence of HCV among the HD patients was 12.7%.37 The most common genotype was 1b, followed by 1a and 2a, and finally by 2b and 2c. The authors detected no patients with genotypes 3-6.38 A multivariate analysis showed that the risk factors for hepatitis C were transfusions before the year of 1991; excluding the year of 1991, the analysis showed that a history of surgery and prolonged time under HD were all significantly associated with the presence of hepatitis C.37 In 2004, Méndez-Sánchez, et al. reported the prevalence of HCV in HD patient at a tertiary care hospital. They studied 149 patients in HD with a mean age of 51 ± 17 years, 53% of whom were male. The prevalence of anti-HCV antibodies was 6.7%, and viremia was detected in eight out of 149 (5%) patients. The most common genotype was 1a, followed by 1b. The main causes of kidney failure were diabetic nephropathy, reflux nephropathy, and glomerulonephritis.39 In the Mexican population, the prevalence of infection by the HCV decreased from 10.2% in 2000 to 6.7% in 2004.
PeruThe incidence rate of end-stage chronic renal disease in Peru is 34.3 pmp, with a prevalence of HD of 230.7 pmp.14 The prevalence of hepatitis C has been reported as being between 60 and 90% in Peruvian HD centres. In March of 2000, Cieza, et al. described risk factors for HCV infection based on a univariate analysis; they included the number of blood transfusions and time in HD (P < 0.05). A multinomial logistic regression analysis revealed that the only variable that represented a risk for HCV infection was blood transfusion (OR = 4.8; 95% CI, 1.6-14.4).40 In 2005, Méndez-Chacón, et al. detected anti-HCV antibodies in 76 out of 128 (59%) HD patients. The annual seroconversion rate was 13% (6/48). A positive serology for HCV was found in 56% of patients who received one to three transfusions, in 66% of patients who received four to nine transfusions and in 85% of patients who received more than 10 transfusions. Patients with a positive serology averaged 54 months of permanence in HD compared with seronegative patients, who received an average of 26 months of HD.41 The more common genotype was genotype 1. A large study found that time on HD (OR = 7.13; 95% CI, 3.04-17.02), more than two hospitalizations (OR = 4.49; 95% CI, 1.2817.28), treatment at multiple HD centres (P < 0.5), having undergone a transplant (P < 0.01) and having received a blood transfusion (OR = 2.61; 95% CI, 1.04-6.68) were factors that were associated with HCV infection.42
Other CountriesThe incidence rate of end-stage chronic renal disease in Colombia is 141.6 pmp, with a prevalence of HD of 306.5 pmp.14 An assessment performed at a single centre in that country revealed a prevalence of hepatitis C in multi-transfused HD patients of 6.1%, and the main risk factor associated with infection by HCV was the reception of transfusions before 1995.43
The incidence rate of end-stage chronic renal disease in Uruguay is 161.0 pmp, with a prevalence of HD of 671.5 pmp.14 A study of 409 patients conducted in that country showed that 64 (15.6%) patients were on HD and reported a prevalence rate of HCV among patients undergoing HD of 6.3%. There was a direct relationship between the number of products transfused and the prevalence of both hepatitis C antibodies and HBcAb.44
In Venezuela, the prevalence of patients in HD is 339.8 pmp,14 and the prevalence of HCV in HD in 1996 was 71%.45 In 2012, Monsalve-Castillo, et al. found that the risk factors for acquiring HCV infection/HCV seroconversion were 0.3270 (95% CI, 0.01323-8.080) in HD patients in Venezuela. These findings suggest a lack of significant sources of HCV infection because of the preventive measures used to avoid its transmission in the HU.46
ConclusionsWe found that the prevalence of HCV infection in HD patients ranged from 4.2 to 83.9%, which was greater than that detected in developed countries (Table 2). The most common genotype was genotype 1, and subtype 1b was most prevalent, followed by genotype 1a. The risk factors associated with HCV in HD patients were the duration of the HD treatment (suggesting that a longer HD permanence time implies a higher probability of acquiring HCV), blood transfusions, surgeries, having undergone a transplant, pre-existing liver disease, higher ALT levels and intravenous drug abuse (Table 3). RTPCR is crucial for the diagnosis of HCV infection in HD patients. Trials using combinations of new oral antiviral drugs, such as sofosbuvir and combo (ombitasvir, paritaprevir, ritonavir and dasabuvir and Grazoprevir), should be the next step in the improvement of the care of HD patients with HCV, because they apparently do not require dose adjustment according to renal function.11,47,48
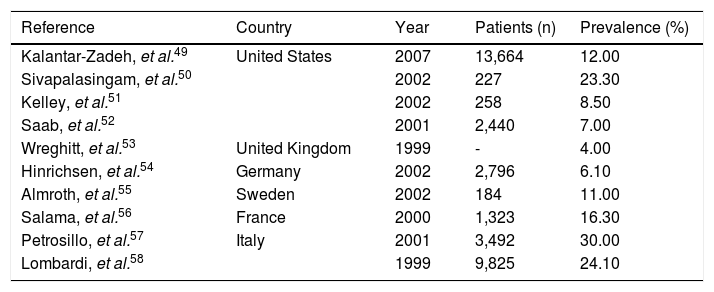
Prevalence of anti-hepatitis C virus seropositivity in haemodialysis patients in developed countries.
| Reference | Country | Year | Patients (n) | Prevalence (%) |
|---|---|---|---|---|
| Kalantar-Zadeh, et al.49 | United States | 2007 | 13,664 | 12.00 |
| Sivapalasingam, et al.50 | 2002 | 227 | 23.30 | |
| Kelley, et al.51 | 2002 | 258 | 8.50 | |
| Saab, et al.52 | 2001 | 2,440 | 7.00 | |
| Wreghitt, et al.53 | United Kingdom | 1999 | - | 4.00 |
| Hinrichsen, et al.54 | Germany | 2002 | 2,796 | 6.10 |
| Almroth, et al.55 | Sweden | 2002 | 184 | 11.00 |
| Salama, et al.56 | France | 2000 | 1,323 | 16.30 |
| Petrosillo, et al.57 | Italy | 2001 | 3,492 | 30.00 |
| Lombardi, et al.58 | 1999 | 9,825 | 24.10 |
Risk factors for infection with hepatitis C virus in haemodialysis patients in Latin America.
| 1. Longer haemodialysis treatment time.18,20,21,24,27,29,33,37,38,42 |
| 2. Transfusion before 1991;37,38 1992;22 1 99 5.43 |
| 3. Others: blood transfusion, more than two hospitalizations, treatment at multiple haemodialysis centres, having undergone a transplant, pre-existing liver disease, higher ALT levels and intravenous drug abuse. |
ALT: alanine aminotransferase.
- •
ALP: alkaline phosphatase.
- •
ALT: alanine aminotransferase.
- •
CAPD: continuous ambulatory peritoneal dialysis.
- •
CI: confidence interval.
- •
CKD: chronic kidney disease.
- •
ELISA: enzyme-linked immunosorbent assay.
- •
HbcAb: hepatitis B core antibody
- •
HbsAg: hepatitis B surface antigen.
- •
HCV: hepatitis C virus.
- •
HD: haemodialysis.
- •
HU: haemodialysis unit.
- •
OR: odds ratio.
- •
pmp: per million persons.
- •
RT-PCR: reverse transcription polymerase chain reaction.
- •
UI: uncertainty interval.
- •
UMELISA: ultramicroenzyme-linked immunosorbent assay.
- •
WHO: World Health Organization.
This Study was Supported by Medica Sur Clinic & Foundation.
DisclosuresAuthors have no conflict of interest to declare.