Introduction. Drug-induced liver injury (DILI) remains a major problem for drug development and represents a challenging diagnosis for clinicians. The absence of specific biomarkers for diagnosing DILI precludes the availability of reliable data on the epidemiology of the disease. In this study we aimed to describe the features of idiosyncratic hepatotoxicity reports in Latin American countries.
Material and methods. A literature search was performed using the online version of MEDLINE, EMBASE, Scopus, Google Scholar and specific data bases from Latin America (LA) (Scielo, Lilacs) to identify any case report or case series of published DILI from 1996 to 2012. From 1996 to 2012, a total of 176 patients with DILI were published in LA, involving 53 suspicious drugs. The median age in the adult population of these patients was 55 years (17-82) with prevalence of women (67%). Among main therapeutic classes, the rank order was led by non-steroidal anti-inflammatory (61 cases) and systemic antibacterial drugs (37 cases). Nimesulide was the individual drug responsible for the highest number of cases (53), followed by cyproterone acetate (18), nitrofurantoin (17), antituberculous drugs (13) and flutamide (12). Thirty two percent of published cases evolved to acute liver failure (ALF), and half of the subjects required liver transplantation or eventually died.
Conclusions. This study represents the first structured attempt to assess the spectrum of DILI profile in LA. The establishment of a Latin American registry to collect prospective DILI cases using a standardized protocol will advance our knowledge about idiosyncratic DILI in this region.
Drug-induced idiosyncratic liver injury (DILI) remains a major concern for pharmaceutical companies leading to high attrition rates during drug development and adoption of post-marketing regulatory measures. It represents a challenging diagnosis for clinicians and hepatologists and has been the most frequent single cause of safety-related drug marketing withdrawals in the past 50 years. Indeed, DILI is an intriguing condition that occurs with drugs otherwise commonly used and tolerated by most subjects. Despite growing research efforts in this field the mechanisms and host factors that render individuals susceptible to toxicity of a given drug are still not fully understood. The DILI clinical scenario encompasses a wide spectrum of phenotypes and severity. Of note, DILI is the most common cause of acute liver failure (ALF) in Western countries.1
Lacking specific biomarkers for DILI diagnosis precludes the availability of reliable data on the epidemiology of this condition, as its diagnosis is generally made by exclusion of other causes of liver disease and relies heavily on clinical suspicion. Therefore, severe cases may be overestimated while milder or adaptive forms of injury may be under-diagnosed.2
To overcome such limitations, and to better understand the causative drugs, risk factors and outcomes of DILI cases, country-based registries and multicenter research networks have been established. In countries such as Sweden since 1970 (Swedish Drug Reaction Advisor Committee, SDRAC),3 and prospectively in Spain since 1994 (Spanish DILI Registry),4 and the United States since 2003 (Drug Induced Liver Injury Network, DILIN).5 These multicenter registries have fostered case identification and recruitment following a structured protocol, and have stimulated consensus for case definition and phenotype classification.2,6
Since existing registries cover restricted geographical areas from North America and Europe, similar efforts are being conducted in other regions such as LA. However, epidemiological information on DILI in LA is still lacking. In this study, we aimed to describe the features of idiosyncratic hepatotoxicity reports in several countries in LA by retrieving, and analyzing all published cases from this region in the last sixteen years.
Material and MethodsA literature search was performed using the online version of MEDLINE, EMBASE, Scopus, Google Scholar and specific data bases from LA (Scielo, Lilacs) to identify any case report or case series of published DILI from 1996 to 2012. The medical literature was searched using the terms: “liver injury”, “hepatotoxicity”, “drug-induced cholestasis”, “fulminant liver failure”, “acute liver injury”, “drug-induced liver injury”, “acute liver failure”. These were combined with the Boolean set operators “AND” and “OR”. There were no language, species and article type restrictions. The keywords were checked in all fields of these data base records.
Web pages of regulatory drug-surveillance centers from different countries were searched for: ANMAT, Argentina; ANVISA, Brazil; COPREFIS, Mexico; MSP, Uruguay; INVIMA, Colombia; CEFARVI, Venezuela; CENIMEF, Chile: DIGEMID, Peru; SNFV, Bolivia; UNCFV, Cuba. All published and available full text cases were retrieved. Only published DILI cases linked to drugs were included in the study, regardless of whether standardized diagnostic scales for causality evaluation were applied or not. Instances involving herbal and complementary medicines instances were excluded. With some exceptions, publications prior to 2000 were not available in electronic format. Data from Colombia’s series of cases could not be analyzed due to missing data relevant to case descriptions.7
Information was retrieved regarding the country source of the report, the patient’s demographic data, suspected drug and co-medications and the outcome of the reaction.
Some liver transplant centers from LA countries were contacted in order to obtain information related to cases developing ALF associated with drugs.
The drugs considered to be implicated in liver damage were classified according to the Anatomical Therapeutic Classification of the World Health Organization (ATC/DDD Index 2013).8
Statistical analysisVariables were examined using descriptive statistics.
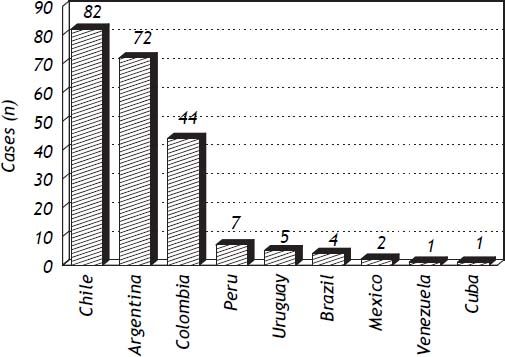
ResultsLatin American countries with identified publications of hepatotoxicity cases were Argentina, Brazil, Colombia, Cuba, Chile, Mexico, Peru, Uruguay and Venezuela, as shown in figure 1.
From 1996 to 2012, a total of 176 cases (including 10 pediatric cases) with a DILI episode, involving 53 suspicious drugs, were published in LA. (9-33) Of the 53 drugs involved, 24 were responsible for at least two cases (Table 1). In 13 cases (7%) more than one drug was involved, twelve cases involved two drugs and one case involved three. The median age of the adult population was 55 years (17-82), with a prevalence of women in 112/166 cases (67%). Imaging data were available in 76% of the publications. As regards virological tests, HBV, HCV, HAV, CMV, EBV, HSV, HEV were recorded in 72, 68, 64, 28, 24, 8 and 4% of the cases, respectively. The authors state alcohol history in 28% of the reports and auto antibodies titres are provided in 72% of them. Liver histology was available in 79 cases (8 explanted liver and 3 postmortem). A positive re-challenge was described in 11 cases (6.25%).
Causative drugs in case reports or series of drug-induced liver injury published in Latin America between 1996 and 2012. (N patients = 176).
| Culprit drug (n = 53) | Number of cases published (n = 191)* | Clinical data | Country/year/ref | Number of cases/drugs |
|---|---|---|---|---|
| Nimesulide | 43 | 6 ALF (14%), 1 OLT, 2†, 60 y, | Argentina, 20109 | 53 |
| 5 | 35 F, 1R 5 ALF, 5 F, 2†, 1 OLT | Argentina (unpublished data) | ||
| 5 | 2 ALF, 2†, 61 y, 5 F, 1R | Uruguay, 199810 | ||
| Cyproterone acetate | 18 | 7 ALF, 3†, 73 y, 18 M, 2R | Argentina, 201111 | 18 |
| Nitrofurantoin | 12 | 3 ALF, 1†, 55 y, 12 F | Chile, 201212 | 17 |
| 3 | No ALF | Chile, 200313 | ||
| 2 | 2 ALF, 2F | Chile, 201214 | ||
| Flutamide | 1 | Cholestasis, 71 y, M, R | Colombia, 200915 | 12 |
| 10 | 5 ALF, 5 OLT, 43 y, 7 F | Chile, 201116 | ||
| 1 | ALF, F | Chile, 201214 | ||
| Isoniazid | 1 | Hepatocellular, 6 months, M | Brazil, 200017 | 13 |
| 2 | 1 + Rifampicin and Pyrazinamide and 1 + Methotrexate | Chile, 200313 | ||
| 3 | 3 ALF, 3 F | Chile, 201214 | ||
| Pyrazinamide | 1 | + Isoniazid and Rifampicin | Chile, 200313 | |
| Rifampicin | 1 | + Isoniazid and Pyrazinamide | Chile, 200313 | |
| Anti-tuberculosis | 5 | 1 ALF, 1t, 8 y, 3 F, 5R | Peru, 200518 | |
| Methotrexate | 6 | 1 + Retinoid, 1 + Isoniazid | Chile, 200313 | 6 |
| Propylthiouracil | 1 | ALF, †, 51 y, F | Cuba, 200819 | 5 |
| 2 | 1 ALF, 1† | Chile, 200313 | ||
| 2 | 2 ALF | Chile, 201214 | ||
| Piroxicam | 2 | 1 + Dipyrone | Chile, 200313 | 5 |
| 1 | 22 y, F, cholestasis | Chile, 199820 | ||
| 2 | 1 F, 44 y, ALF; 1 M, 42 y, cholestasis | Argentina, 201021 | ||
| Halothane | 1 | ALF, OLT, 35 y, F, R | Colombia, 200122 | 5 |
| 2 | 2 ALF, 2†, 1 + Paracetamol | Chile, 200313 | ||
| 2 | 2 ALF | Chile, 201214 | ||
| Valproic acid | 3 | 1 ALF, 1†, 4 y, 2 M | Brazil, 199623 | 3 |
| Amoxicillin-clavulanate | 1 | Cholestasis, 72 y, M | Chile, 199924 | 3 |
| 1 | Hepatocellular, 27 y, M | Mexico, 201125 | ||
| 1 | No ALF | Chile, 200313 | ||
| Phenytoin | 1 | + Benzathine Penicillin | Chile, 200313 | 3 |
| 2 | 2 ALF, 2 F | Chile, 201214 | ||
| Ketoconazole | 1 | No ALF | Chile, 200313 | 3 |
| 2 | 2 ALF, 2 F | Chile, 201214 | ||
| Diclofenac | 1 | No ALF | Chile, 200313 | 2 |
| 1 | ALF, F | Chile, 201214 | ||
| Dipyrone | 2 | 1 + Estradiol /Prasterona 1 + Piroxicam | Chile, 200313 | 2 |
| Ethinyl-Estradiol/ Levonorgestrel | 2 | 1 + Cotrimoxazole | Chile, 200313 | 2 |
| Lamivudine | 2 | + Zidovudine | Chile, 200313 | 2 |
| Methyldopa | 1 | No ALF | Chile, 200313 | 2 |
| 1 | ALF, F | Chile, 201214 | ||
| Propafenone | 2 | Cholestasis, 68 y, 2 F | Argentina, 200326 | 2 |
| Retinoids | 2 | 1 + Methotrexate | Chile, 200313 | 2 |
| Terbinafine | 1 | Hepatocellular, 53 y, F | Mexico, 200327 | 2 |
| 1 | Cholestasis, 31 y, F | Peru, 200428 | ||
| Zidovudine | 2 | + Lamivudine | Chile, 200313 | 2 |
| Amitriptyline | 1 | + Carbamazepine | Chile, 200313 | 1 |
| Anaproline (Nandrolone) | 1 | No ALF | Chile, 200313 | 1 |
| Carbamazepine | 1 | + Amitriptyline | Chile, 200313 | 1 |
| Cyclophosphamide | 1 | + Procarbazine | Chile, 200313 | 1 |
| Claritromycyn | 1 | ALF, F | Chile, 201214 | 1 |
| Chlormezanone | 1 | No ALF | Chile, 200313 | 1 |
| Chlorpromazine | 1 | No ALF | Chile, 200313 | 1 |
| Sulfamethoxazole/ Trimethoprim | 1 | + Ethinyl- Estradiol/ Levonorgestrel | Chile, 200313 | 1 |
| Naproxen | 1 | ALF, F | Chile, 201214 | 1 |
| Disulfiram | 1 | ALF, F | Chile, 201214 | 1 |
| Estradiol/prasterona | 1 | + Dipirone | Chile, 200313 | 1 |
| Enalapril | 1 | + Ticlopidine | Chile, 200313 | 1 |
| Griseofulvin | 1 | ALF, F | Chile, 201214 | 1 |
| Imatinib | 1 | ALF, †, 59 y, F | Argentina, 200729 | 1 |
| Mebendazole | 1 | No ALF | Chile, 200313 | 1 |
| Mycophenolate | 1 | ALF, F | Chile, 201214 | 1 |
| Nevirapine | 1 | ALF, M | Chile, 201214 | 1 |
| Paracetamol | 1 | ALF, †, + Halothane | Chile, 200313 | 1 |
| Benzathine Penicillin | 1 | + Phenytoin | Chile, 200313 | 1 |
| Procarbazine | 1 | + Cyclophosphamide | Chile, 200313 | 1 |
| Progesterone | 1 | ALF, F | Chile, 201214 | 1 |
| Gold salts | 1 | Cholestasis, 37 y, F | Peru, 200430 | 1 |
| Tamoxifen | 1 | ALF, F | Chile, 201214 | 1 |
| Ticlopidine | 1 | + Enalapril | Chile, 200313 | 1 |
| Trovafloxacin | 1 | ALF, †, 52 y, F | Venezuela, 200031 | 1 |
| Verapamil | 1 | Cholestasis, 62 y, F | Chile, 201032 | 1 |
| Vitamin A | 1 | Chronic Hepatitis, 25 y, M | Argentina, 200633 | 1 |
ALF: acute liver failure. OLT: orthotopic liver transplantation. AH: acute hepatitis.
The main causative pharmacologic group of drugs was skeletal muscle (32%), followed by anti-infectious (19%), and genito urinary system and sex hormones (18%). Among the main therapeutic class, the rank order was non-steroidal anti-inflammatory drugs (NSAID) (61 cases) and systemic antibiotics (37 cases) (Table 2). Nimesulide was the individual drug responsible for the highest number of cases (53), followed by cyproterone acetate (18), nitrofurantoin (17), antituberculous drugs (13) and flutamide (12).
Rank order of the main therapeutic classes involved in cases of suspected DILI published in Latin America between 1996 and 2012.
| Therapeutic class | n (%) | ALF-OLT n (%) † |
|---|---|---|
| Non-steroidal anti-inflammatory and anti-rheumatic drugs (Nimesulide*, Piroxicam*, Gold salts, Diclofenac*, Naproxen*) | 62 (32) | 16 (26) |
| Anti-infectious (Nitrofurantoin,* Isoniazid,* Pyrazinamide, Rifampicin, Amoxicillin clavulanate, Trovofloxacin,* Sulfamethoxazole/Trimethoprim, Benzathine penicillin, Clarytromycin*) | 37 (19) | 11 (30) |
| Genito urinary system and sex hormones Sexual Hormones (4) (Ethinyl -estradiol/levonorgestrel, estradiol/Prasterone, Progesterone*) Anti-androgens (30) (Cyproterone acetate,* Flutamide*) | 34 (18) | 14 (22) |
| Antineoplastics and immunomodulators (Methotrexate, Imatinib,* Cyclophosphamide, Procarbazine, Tamoxifen*) | 10 (5) | 2 (3) |
| Anticonvulsivants (Valproic acid,* Carbamazepine, Phenytoin*) | 7 (4) | 3 |
| Cardiovascular system (Propafenone, Verapamil, Enalapril, Methyldopa*) | 6 (3) | 1 |
| Anti-virals (Zidovudine, Lamivudine, Nevirapine*) | 5 (3) | 1 |
| Anesthetics (Halothane*) | 5 (3) | 5 |
| Antithyroid (Propylthiouracil*) | 5 (3) | 4 |
| Other groups: analgesics (Acetaminophen/Paracetamol, Dipyrone), retinoids (Retinoids), antifungal agents (Terbinafine, Ketoconazole*, Griseofulvin*), vitamins (Vitamin A), antihelmintic (Mebendazole), psychoanaleptics (Amitriptyline), psycholeptics (Chlorpromazine), anti-thrombotic (Ticlopidine), anabolic steroidal (Anaproline/Nandrolone), muscular relaxants (Chlormezanone), other nervous system drugs (Disulfiram*), immunosuppressants (Mycophenolate*). | 20 | 5 |
| Total | 191 | 62 (32) |
Thirty two percent of published cases evolved to ALF, and half of them required liver transplantation or the patient eventually died. Causative drugs more frequently implicated in ALF were flutamide (6/12, 50%), followed by cyproterone acetate (7/18, 39%) and nimesulide (13/53, 25%).
The largest DILI series published analyzed in this study came from a Medellin Hospital (Colombia).7 The authors identified 42 DILI patients between 2001 and 2008, with a mean age of 41 years (0.4-67) and poly-medicated (67%). Causality was assessed by applying the Maria and Victorino scale.34 Four patients received doses above the therapeutic range. The drugs most commonly reported as responsible for liver injury were antibiotics (36%), followed by anticonvulsants (12%), immunosuppressive agents (9.5%) and NSAIDs drugs (7.2%) and acute hepatitis was the most frequent presentation (38%). Eight patients (20%) went on to develop ALF, while only 9.8% of them underwent liver transplantation.
Using a different methodological approach, Chilean authors analyzed 57 cases that had liver biopsies performed under suspicion of hepatotoxicity during a period of 12 years (1988-2000), and found 33 DILI cases in which the Maria and Victorino scale34 was used for causality assessment. The average age was 48 years (20-76) with a predominance of cholestatic liver injury (30%), hepatocellular injury being present in only 24% of the cases biopsied. ALF was observed in 9% of cases. Similar to the Colombian study described above, antibiotics ranked first as the causative group.13
With the exception of the two series mentioned above (assessing causality by the Maria and Victorino),7,13 and the cyproterone series,11 where the CIO-MS/RUCAM scale was applied,34 none of the other published series or cases stated applying any diagnostic scale.9,10,12,14–33
Countries that provide liver transplantation centers in LA are: Argentina, Brazil, Colombia, Costa Rica, Cuba, Chile, Ecuador, Mexico, Peru, Venezuela and Uruguay. A survey in Argentina (Villamil F, personal communication) of 206 adults and 219 children with ALF found that hepatotoxicity accounted for 12 and 1.4% of the cases, respectively. It is worth mentioning that no liver toxicity due to acetaminophen overdose was identified among the ALF cases. Spontaneous survival in adults was 20%, which is lower than the figure for viral etiologies. In Chile, the experience of two centers in the last 10 years showed that 32% of 129 ALF cases corresponded to idiosyncratic hepatotoxicity, with paracetamol accounting for 5.4% of the cases.14
DiscussionThe true incidence of DILI in LA remains unknown. A prospective study carried out in France found an incidence of DILI of 14/100,000 inhabitants per year, which is in the same range as the incidence of viral hepatitis.36 More recently, Björnsson, et al. (2013) found in Iceland an annual crude incidence of DILI of 19.1/100.000 inhabitants.37 Since prospective population based studies are difficult to conduct, alternative approaches such as the organization of local DILI registries have been set up in other areas of the world in order to gain knowledge on the epidemiology of DILI.38A simpler way to estimate the relevance of DILI is to analyze published reports in the literature from a given area or country. This approach does not allow for obtaining figures of DILI incidence but at least reflects the general characteristics of the problem in the geographic area where the cases are identified. The present study examined published case reports and case series related to hepatotoxicity in LA in a time frame of 16 years. An important finding of this analysis has been the dissimilarities in the reporting of DILI cases among countries, since the bulk of the reports came from Chile, Argentina and Colombia, altogether accounting for 91% of all cases reported. The reason for these major differences is unclear, but it could include more awareness of the groups reporting cases in these countries, as well as the exclusion of herbal products and alternative medicines from the analyses, which could represent a leading cause of DILI in countries such as Brazil and Mexico.39
Non-steroidal anti-inflammatory and anti-infectious drugs were the most frequently reported therapeutic group of causative drugs in LA. In the prospective study conducted by Björnsson, et al., amoxicililin-clavulanate and diclofenac were the most frequently agents implicated (22 and 6%, respectively).37 While anti-infectious drugs predominate in existing registries, NSAIDs are variably represented.3–5 Interestingly, in an international collaborative work that compared drugs by study groups in Spain, Sweden, and the USA as causes of DILI,40 only 31 of the 385 drugs listed (9.6%) were present in the three registries.
The over-representation of NSAIDs in this study can be explained at least in part by the publication of the largest series of nimesulide instances of hepatotoxicity by Bessone et al. in Argentina.9
Interestingly, the antiandrogen drug cyproterone acetate (10%) ranked second as responsible for DILI instances in the series published in LA, despite its rarity or absence in other sources and databases. Its high prevalence in this study is mainly due to the large cyproterone series reported by Bessone, et al.11 Indeed, cyproterone acetate is not included among the list of drugs most commonly related to hepatotoxicity in the different registries.40 On the contrary, cyproterone acetate is frequently related to ALF.41 Flutamide, another antiandrogen, was one of the drugs most often identified as causal in hepatotoxicity, highlighting the impact of the large series of severe cases reported by Brahm, et al. from Chile.16
DILI cases associated with cyproterone acetate and nitrofurantoin, which ranked third in this compilation, were frequently reported in the publications retrieved, however, they are seldom reported in other countries.40 This finding highlights the differences in pharmaceutical policies and prescription patterns among LA and other areas. As well, this epidemiological approach to DILI analysis has allowed for obtaining information relating to safety or benefit/risk concerns that ultimately may facilitate regulatory decision-making.
Indeed, three drugs identified in the present study, namely nimesulide, chlormezanone and trovafloxacin, have been withdrawn from the pharmaceutical market in Spain due to hepatotoxicity.42 With regard to nimesulide, regulatory measures have also been taken in LA, including its withdrawal from the market in Argentina,43 and the reduction of the maximum prescribable dose to 100 mg in Uruguay.
It is worth mentioning the difficulties in obtaining detailed information to ascertain causality. These difficulties were recently discussed by Agarwal, et al.,44who analysed 97 published hepatotoxicity cases relating to six causative drugs. Many essential diagnostic elements were under-reported and the minimal data needed to determine the causes of the adverse effects were not provided. In the present analysis we could verify this serious problem (for example: one third of the cases have not ruled out HAV, and in most of them EBV and HEV were not ruled out). A positive rechallenge to a suspect drug is considered the most robust evidence for DILI diagnosis, but is rarely performed on-purpose due to life-threating risks which pose an ethical concern.
Indeed, although the authors in their respective publications pointed out the type of liver injury, this item was not analysed in the present study since definitions used were heterogeneous. Recently, an international group of experts published a consensus document setting up definitions and phenotype standardization in DILI6 that should be mandatory to follow if comparative analyses are to be performed.
Sixty-two (32%) of the 191 DILI cases considered in this work involved ALF, and were ascribed to 22 medications. The design of this study could explain the overrepresentation of ALF cases in comparison to other case series included in registries,3–5 as there may have been a selection bias in the reporting with a tendency to publish more severe cases or cases with atypical presentation.
Again, NSAIDs were responsible for the highest number of ALF cases, although the relative frequency of ALF was greater for cyproterone acetate. This data would probably be an overestimation because of the large nimesulide series reported,9 representing 11 of the 16 ALF cases related to NSAIDs. Even when NSAIDs have shown progression to liver failure, these cases are less frequently described.41
This LA casuistic analysis of ALF cases is quite different from the reality of other series from USA or Europe. A recent analysis of the US-based UNOS (United Network for Organ Sharing) database from 1987 to 2006 showed that of 661 patients transplanted because of ALF due to DILI, acetaminophen was the first causative medication (40%), followed by antituberculous drugs (8%), antiepileptic (7%) and then antibiotic (6%) medications.45 The NIH ALF group analysis, recently updated with a large number of patients (n = 1198), demonstrated that 11.1% of ALF corresponded to idiosyncratic hepatotoxicity, antibiotics being the main related group (46%), with female predominance (71%) and a low free-of-transplantation outcome (27%).41
Physicians, authors, reviewers, editors all need to understand the importance of establishing the most likely or probable cause of liver problems observed and providing the clinical information needed for making the medical differential diagnosis. Just finding an “association” is not sufficient. Indeed, there are cases that are being published and made available to the scientific community lacking minimal elements to reliably ascertain causality -mainly a consistent time sequence and carefully exclusion of other liver diseases causes. As there is no specific biomarker for DILI, a positive rechallenge provides available evidence and has become the gold standard for diagnosis.46 The application of a specific diagnostic tool for causality assessment before and adverse reaction is accepted for publication and particularly the CIOMS/RUCAM scale which provides a framework that highlights the topics to be addressed in cases of suspected hepatic adverse reaction to improve the consistency of judgments, would help to obtain a more accurate DILI diagnosis.47 Educational programs on DILI clinical diagnosis and management are being implemented by the LA group in order to expand knowledge on this complex and intriguing disorder.
Latin America comprises more than 20 countries with a variety of ethnic groups. Thus, it is very likely that there are significant differences in DILI features, both within the LA region and between it and the rest of the world. These potential differences in DILI susceptibility may be relevant for the introduction of new drugs in the area and therefore deserves characterization. A panLatin American registry of hepatotoxicity is currently being organized with the support of the Spanish DILI Registry (www.slatindili.uma.es) as hepatotoxicity registries are the ideal instrument for systematic and continual data collection, using preestablished criteria based on consensus, which ultimately stimulate research and improve understanding of the complex mechanism underlying this disease.48 This ambitious project is expected to provide a more realistic picture of idiosyncratic hepatotoxicity in LA and will contribute to conducting more robust phenotypic and genotypic studies of DILI in this area of the world.
Abbreviations- •
ALF: acute liver failure.
- •
CIOMS: Council for International Organizations of Medical Sciences.
- •
CMV: cytomegalovirus.
- •
DILI: drug-induced liver injury.
- •
EBV: Epstein Barr virus.
- •
HAV: hepatitis A virus.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HEV: hepatitis E virus.
- •
HSV: herpes simplex virus.
- •
LA: Latin America.
- •
NSAID: non steroidal anti-inflammatory drugs;
- •
OLT: orthotopic liver transplantation.
- •
RUCAM: Roussel Uclaf Causality Assessment Method.
Supported in part by a research grant from the Spanish Agency of Medicines and Medical Devices and the Asociación Universitaria Iberoamericana de Postgrado (AUIP). CIBERehd is funded by the Instituto de Salud Carlos III.