The tumor suppressor PTEN is a phosphoinositide phosphatase regulating the PI3K/Akt signaling pathways and mutated or deleted in a variety of human cancers. Recent evidence indicates that dysregulated PTEN expression and activity in the liver critically affect hepatic insulin sensitivity and trigger the development of non-alcoholic fatty liver diseases. As well, PTEN expression/activity is also affected with HBV and HCV infection, or following alcohol-related injury. Finally, PTEN mutations/deletions or low PTEN expression are associated with diverse liver malignancies thus suggesting a critical role for PTEN in hepatic cancers. This review will focus on our current knowledge of the regulation of PTEN expression/activity and the role of this phosphatase in liver diseases.
The PI3K/Akt signaling pathway plays an important role in the mechanisms by which numerous cell surface receptors control crucial cellular functions including nutrients metabolism, cellular growth, proliferation and apoptosis. Dysregulated signaling through the PI3K/Akt axis is now a well-established prominent feature of metabolic diseases and cancer. In particular for the liver, a growing body of evidence indicates that aberrant PI3K/Akt signaling importantly contributes to liver dysfunctions and pathologies associated with the metabolic syndrome, viral infections, alcohol abuse and in hepatocellular adenoma (HCA) and carcinoma (HCC).
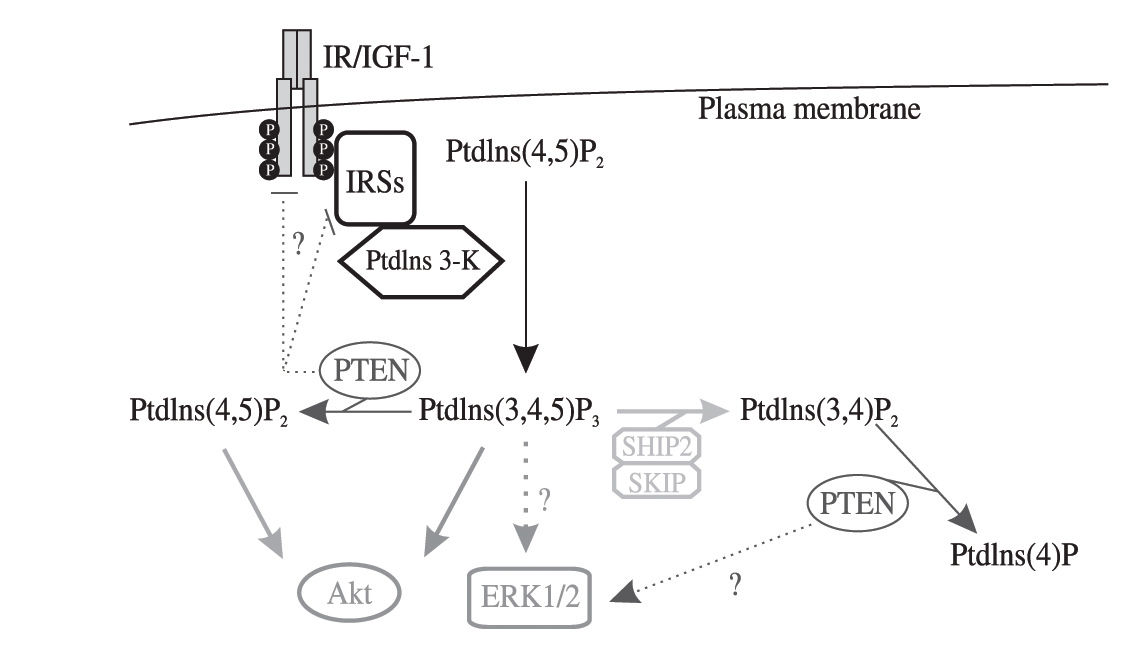
The intensity and specificity of PI3K-dependent signaling is tightly regulated in time and space by specific enzymes and complex molecular mechanisms.1-2 In these regulatory processes, PTEN (phosphatase and tensin homolog deleted on chromosome 10) acts as a potent negative regulator of PI3K signaling. Indeed, PTEN is a dual activity phosphatase, being capable to dephosphorylate both proteins and PtdIns(3,4)P2/PtdIns(3,4,5)P3, the second messengers generated by PI3K activation3(see Figure 1) PTEN was originally identified as a tumor suppressor gene 4 frequently mutated or deleted in a variety of human cancers,5-7 but its role also as a negative regulator of growth hormones signaling, in particular insulin/IGF-1 signaling, in peripheral tissues such as the liver has been strongly supported by numerous studies (reviewed in [8]). Recent reports have also begun to shed light on the important role that altered PTEN expression/activity plays in promoting the development of metabolic liver disorders and HCC. The aim of this review is to examine the current knowledge of the PTEN role in the development of a wide spectrum of human liver disorders.
Simplified scheme depicting the phosphoinositide phosphatase activity and other potential roles of PTEN in insulin/IGF-1 signaling. Dotted lines and question marks indicate controversial functions of PtdIns(3,4,5)P3 and PTEN in the control of ERK1/2, IRSs and growth hormones receptors expression or activities. PtdIns(3,4,5)P3 dephosphorylation on the 5' position by two other phosphoinositide phosphatases, i.e. SHIP2 and SKIP, which are involved in insulin/IGF-1 signal transduction, is also highlighted.
Consistent with the well described PTEN lipid phosphatase activity (Figure 1), PTEN overexpression in non-hepatic cell models was shown to inhibit insulin-induced PtdIns(3,4)P2/PtdIns(3,4,5)P3 production, Akt activation, GLUT4 translocation and glucose uptake. In contrast, PTEN downregulation by specific siRNA in 3T3-L1 pre-adipocytes enhanced Akt phosphorylation and glucose transport (reviewed in 5). Whether PTEN also regulates insulin-activated mitogenic pathways in vitro or in vivo remains a still controversial issue8(Figure 1) The role of PTEN in cultured hepatocytes for the control of insulin signaling has been poorly investigated, but two studies where PTEN was specifically deleted in the livers of mice demonstrated that loss of PTEN function enhances insulin sensitivity and causes an overall improved glucose tolerance.9,10 Similarly, PTEN antisense oligonucleotides, which reduce PTEN expression in the liver and in the adipose tissue, reversed hyperglycemia in ob/ob mice and normalized plasma glucose in the db/db mice.11 Finally, whole-body insulin sensitivity and glucose tolerance were increased in PTEN-/+ heterozygous mice.12,13
Although genetic depletion of PTEN in the liver, as well as in the muscle and in the adipose tissue,14,15 clearly affected systemic insulin sensitivity in animals, it is less clear whether pathophysiological dysregulation of PTEN expression can occur in the liver and modulate insulin sensitivity and the glucose metabolism. In this regard, we have recently shown that hepatic PTEN expression is significantly reduced in Zucker diabetic fatty (ZDF) rats compared to their lean littermates, in insulin resistant high fat-fed Wistar rats compared to control animals, and in human obese subjects having steatosis.16 Since PTEN expression was shown to be increased in the soleus muscle and in the heart of diabetic rats,17,18 these data suggest differential pathophysiological regulation of PTEN expression in insulin-sensitive organs with the metabolic syndrome.
Surprisingly, our studies demonstrated also that in hepatoma HepG2 cells, PTEN downregulation increases basal levels of Akt activity, but desensitizes cells for further insulin stimulation by decreasing the expression and phosphorylation of insulin receptors and IRS-1.16 These observations were consistent with another study by Lackey et al. showing similar data in various cancer cell lines.19 These findings are in overt contrast with the phenotype of PTEN liver-specific knock-out mice, which overall display an increased sensitivity to insulin, but the reasons for these discrepancies remains currently unclear. Interestingly, a recent study indicated that in PTEN heterozygous mice, hepatic glucose uptake is restricted due to low glucokinase expression, and that the glucose is diverted from the liver to muscles for increased uptake, thus resulting in overall enhanced insulin sensitivity.12
PTEN in non-alcoholic fatty liver diseases (NAFLD)NAFLD are commonly associated with obesity and diabetes20 and encompass histological features ranging from hepatic steatosis, steatohepatitis, fibrosis and cryptogenic cirrhosis.21 HCC might then occur as a likely end stage of NAFLD and thus NAFLD might be regarded as a preneoplastic state of the liver.
The current concept in the development of NAFLD involves a two hits model, in which the first hit would lead to hepatic steatosis, and the second to non-alcoholic steatohepatitis (NASH). Insulin resistance in hepatocytes may be responsible for both hits, whereas oxidative stress, mitochondrial dysfunctions and dysregulated cytokines signaling are likely critical events to progress toward NASH.22 Further aberrant activation of hepatic stellate and Kupfer cells importantly contribute to the development of liver fibrosis and inflammation.23
Accumulating evidence suggest that PTEN is a critical factor involved in the development and progression of NAFLD to different stages. The more compelling evidence came from analyses of liver-specific PTEN knockout mice, which develop steatosis and steatohepatitis at 10-40 weeks of age.9,10,24 We further provide evidence that unsaturated fatty acids (UFAs) trigger hepatic steatosis by downregulating PTEN expression.16 PTEN-deficient hepatocytes displayed increased expression of lipogenic enzymes fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC)10 and microarray analysis revealed an upregulation of several genes involved in steatosis and inflammation.25 We also recently demonstrated that PTEN downregulation by fatty acids, or PTEN siRNAs, in hepatoma HepG2 cells triggers the development of steatosis, whereas PTEN overexpression in cells exposed to fatty acids prevents lipid accumulation in HepG2 cells.16 These data were strongly supported by analyses of different rat models with the metabolic syndrome and human liver biopsies of obese subjects showing that PTEN downregulation correlated with the presence of steatosis.16 Interestingly, the control of lipid accumulation by PTEN appears to be a phenomenon conserved throughout evolution, since PTEN loss in the fruit fly (Drosophila melanogaster) nurse cells was shown to induce the formation of large lipid droplets.26
Whether hepatic steatosis is strictly associated with hepatic insulin resistance is still a matter of debate. However, cellular pathways transducing the signal of insulin, including the IRS/PI3K/Akt axis, are intimately linked to hepatic lipid metabolism. Of particular interest are the controversial data implicating IRS2 in steatosis development.27,28 Indeed, disruption of IRS2 in mice led to upregulation of lipogenic enzymes and hepatic steatosis, and PTEN was suggested to exert a positive feedback control on IRS2 expression in a breast cancer cell line.28,29
Alteration of PTEN expression/activity is also associated with several cellular defects in non-hepatic cells, but which are implicated in the development of NAFLD. For example, PTEN-dependent dysregulation of TGF β signalling was associated with pulmonary fibrosis.30 Noteworthy, TGF β, which triggers hepatic fibrosis,31 decreases PTEN expression in hepatoma cells.32 In addition, PTEN was shown to regulate Smad4, a common mediator of TGF β in hepatocytes.33 Whether alterations of PTEN expression/activity in injured hepatocytes modulate biosynthesis and secretion of cytokines mediating inflammation, or activation of immune/stellate cells, is also poorly known. However, loss of PTEN was shown to enhance cytokine production in mast cells.34 In a rat model of allergen-induced bronchial inflammation, PTEN was shown to be strongly downregulated in epithelial layers and intratracheal administration of PI3K inhibitors, or ad-enoviruses encoding PTEN, remarkably reduced bronchial inflammation.35 In a follow up study, Lee et al. also demonstrated using the same animal model that PPARγ-mediated anti-inflammatory response correlated with PTEN upregulation.36 Further studies are now required to examine whether PTEN downregulation in hepatocytes with NAFLD leads to excessive secretion of proinflammatory and pro-fibrogenic cytokines.
Finally, mitochondrial dysfunctions, excessive reactive-oxygen species (ROS) production and lipid peroxidation are major metabolic disorders associated with NAFLD and promoting insulin resistance, inflammatory processes, fibrogenesis and apoptosis.37 Functional alterations of mitochondria were shown to occur in steatotic hepatocytes and to favor progression to NASH.38 Consistent with this concept, mitochondrial abnormal morphology with crystalline inclusions is observed in hepatic mitochondria of patients with NASH.39,40 PTEN and a PTEN-like phosphatase (PTPMT1) were found physically associated to mitochondria41,42 and PTEN was shown to play a role in mitochondria-dependent apoptosis.42 Also p53 activity, which regulates PTEN expression/stability,43 is associated with the pathogenesis of hepatic steatosis44 and involved in mitochondrial respiration.45 Whether dysregulation of PTEN expression/activity in fat accumulating hepatocytes might contribute to the insulin resistance and other disorders by altering mitochondrial functions remains, however, to be firmly established.
PTEN in ethanol-induced liver injuryHCC occurrence in developed countries is mostly associated with liver cirrhosis caused by chronic alcohol consumption.46 Ethanol-related liver damages include inflammation, increased iron storage, ROS production via aberrant cytochrome P-450 2E1 activation, mitochondrial damages and apoptosis.47,48 In addition, chronic alcohol exposure leads to hepatocytes regeneration due to the activation of survival signaling factors and interference with the retinoid metabolism.49-52 Given the outcomes of alcohol abuse for the liver and the known PTEN functions, ethanol-induced alterations of PTEN expression/activity in the liver could be expected. In support of this hypothesis, increased apoptosis and decreased insulin signaling in hepatocytes of Long-Evans rats chronically fed with ethanol was associated with increased levels of PTEN mRNA and protein, thus suggesting that ethanol upregulates PTEN expression in the liver.53 Of note, increased hepatic PTEN expression was also observed in rats exposed to alcohol in utero.54 Consistent with these studies, chronic exposure of hepatoma HepG2E47 cells to ethanol increased PTEN expression and subsequently the sensitivity of cells to TNF β-induced cytotoxicity and apoptosis.55 In contrast, ethanol did not affect PTEN expression in Huh-7 hepatoma cells. However, in these cells ethanol increased the physical association between PTEN and the PI3K regulatory subunit p85 α, which functionally resulted in a decreased Akt and downstream effectors activity.56
Together, these studies provide important evidence indicating that ethanol-mediated alterations of PTEN expression or functions could be involved in the mechanisms triggering ethanol-dependent hepatic insulin resistance and cell death.
PTEN and viral hepatitisInfections by hepatitis B virus (HBV) and hepatitis C virus (HCV) are major contributors to the high incidence of HCC occurrence in South-East Asia and Africa.57,58 HCV infection is strongly associated with liver insulin resistance and causes steatosis and fibrosis (reviewed in [59]). In contrast, whether HBV infection causes similar liver disorders remains unclear.60
Few studies have suggested that HBV, or HCV, mediates alterations of PTEN expression and susequent hepatocytes dysfunctions. The Hepatitis B virus-X protein (HBx) was shown to downregulate PTEN mRNA and protein expression leading to uncontrolled Akt activation in Chang liver cells.61 In turn, Akt activation by HBx-mediated PTEN downregulation contributed to enhanced invasive potential of Chang cells by upregulating matrix metallo-proteinase-9 gene expression.62 In accordance with these data, ectopic expression of PTEN was able to reverse pro-survival signaling and inhibition of apoptosis triggered by HBx in Chang cells.63 The same group also demonstrated in a previous report that PTEN prevents HBx-mediated induction of IGF-II expression in hepatoma cell lines.64 Since IGF-II plays an essential role in HCC formation, altogether these data suggest that PTEN downregulation is a critical event in the molecular mechanisms triggered by HBx to induce cell proliferation.
Direct evidence linking a loss or gain of PTEN functions in HCV-mediated liver diseases are scarce. In one study, Waris et al. reported that HCV infection of Huh-7 cells transactivates major regulators of the lipid metabolism, i.e. SREBPs, through mechanisms involving increased cellular oxidative stress and PTEN inactivation by post-translational phosphorylations.65 In another study, correlative immunohistochemical analyses of human HCV-positive cirrhotic HCC indicated that PTEN is downregulated in the tumors and that PTEN expression inversely correlates with expression of iNOS and COX II. High PTEN expression was further described as a prognostic factor for the survival of HCV-positive cirrhotic HCC patients.66
Further molecular, clinical and epidemiological studies are warranted to understand more precisely the mechanisms by which HBV/HCV infections alter PTEN expression or activity in the liver, the pathological outcomes of PTEN dysfunction in HBV/HCV infections, as well as to potentially confirm PTEN expression/activity as a reliable prognostic marker for the stage and gravity of HBV/HCV-related liver diseases.
PTEN and liver malignanciesHepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third cause of tumour-related death in the world.67 HCC develops in the setting of chronic liver diseases, including hepatitis B carrier state, hepatitis C chronic infection, environmental toxins, hemochromatosis, alcoholic cirrhosis and non-alcoholic fatty liver diseases (NAFLD).68 The tumor suppressor PTEN is frequently mutated or deleted in 30% of all human cancers, and is thus, after p53, one of the most common tumor suppressor, which expression/activity is altered during carcinogenesis (reviewed in [69]). Alterations in PTEN tumor suppressor expression/activity appear also to represent a critical factor involved in the occurrence and progression of HCA and/or HCC. In humans, several studies reported weak expression or mutation/deletion of PTEN in HCC70-73 and suggested that PTEN might be an independent prognostic marker for HCC progression.74,75 Studies with animals further supported the important tumor suppressor role for PTEN in the liver. Indeed, PTEN heterozygous mice were shown to develop tumors in multiple organs, including the liver.76 More strikingly, PTEN liver-specific knock-out mice display, at age between 40 and 78 weeks, liver nodular lesions resembling human liver adenomas, which then evolve towards HCC.9,10 Interestingly, a subset of human HCA is also characterized by a marked steatosis77,78 and telangiectatic adenomas, which may progress towards HCC, occurs mainly in overweight/obese patients.79 In this regard, we previously showed that free fatty acids, which are elevated with obesity, decrease PTEN expression in hepatocytes and PTEN expression in human steatotic livers is low.16 However, whether fatty acids promote progression of NAFLD towards the development of HCA/HCC by affecting PTEN expression/activity in the liver has to be further investigated. Finally, PTEN has also been recently implicated in the development of other types of liver malignancies, such as cholangiocellular carcinoma33 and hepatic angiosarcoma.80
At the molecular levels, alterations of PTEN expression/activity have been associated with several cellular defects, in hepatic and non-hepatic cells, promoting carcinogenesis. For example, inhibitors of COX-2, an enzyme highly expressed in HCC,81 restore Fas-mediated apoptosis in human gastric carcinoma cell lines by upregulating PTEN.82 PTEN affects PI3K and MAPK activation by IGF-1/2, which is critical for hepatocyte proliferation,83 and a reciprocal regulation of PTEN and IGF-2 occurs in several cancer cells including hepatoma cells.64,84,85 PTEN is also likely involved in EMT, which favors migration and invasiveness of cancerous cells,86,87 and PTEN expression in the nucleus is essential to maintain chromosomal stability and DNA repair.88 Since EMT and genomic alterations are typical features of HCC,89,90 impaired PTEN expression or activity can represent an important step in progression of NAFLD towards HCC. Interestingly, PTEN also interacts with and dephosphorylates the focal adhesion kinase FAK, thus negatively regulating cell interactions with the extracellular matrix.91 Moreover, FAK activity correlates with the aggressiveness of HCC behavior.92 Finally, PTEN interacts also with E-cadherin/β-catenin adherens junctional complexes and PTEN activity appears to be critical for stabilizing these complexes and for preventing cancer cell invasiveness.93,94 Of note, the Wnt/« •Catenin/Lef-Tcfi signalling pathway plays an important role in the development of hepatic fibrosis, HCA and HCC.95
Although additional studies are now required to convincingly established PTEN as a reliable independent HCA/HCC prognostic marker, there is now solid evidence that mutations or dysregulated PTEN expression/activity strongly promote uncontrolled proliferation and malignancy of hepatocytes.
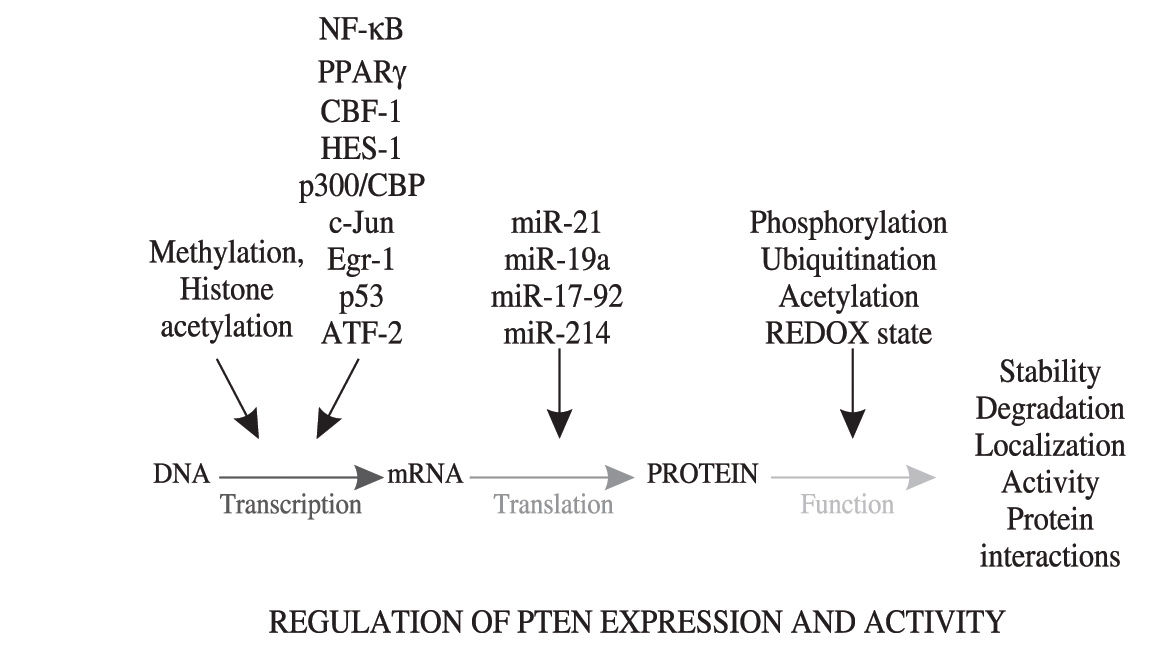
Regulation of PTEN expression/activityDysregulated PTEN expression in hepatocytes, rather than PTEN mutations or deletions, is likely to importantly contribute to the occurrence and/or worsening of several types of liver pathologies. Little is known about the pathophysiological regulation of PTEN expression/activity in the liver, but numerous studies using various cell models have highlighted the complexity of transcriptional and/or post-transcriptional mechanisms regulating the expression and activity of PTEN (see Figure 2) Post-translational modifications such as phosphorylations, ubiquitinations, acetylations and the REDOX state of PTEN can modulate the activity, stability, degradation or sequestration in specific compartments of the protein.96-99 At the transcriptional level, genetic and epigenetic mechanisms controlling PTEN expression have been described. 5’UTR-dependent transcriptional regulation of PTEN mRNA synthesis is under the control of several transcription factors including NF-» B,100 PPAR-γ101 CBF-1,102 p300/CBP,100 c-Jun,103 Egr-1,104 p53,43 HES-1105 and ATF-2.106 Epigenetic PTEN silencing by hypermethylation of its promoter has also been suggested as a potential mechanism contributing to PTEN downregulation in cancer, but whether this process is dysregulated in NAFLD is yet unknown.69,107 In addition, hypermethylation of the PTEN promoter is encountered only in 16-17% of human primary liver HCC.109,110 Of interest, histone deacetylases inhibitors, an emerging new class of potential anti-cancer agents against hepatocellular carcinoma,111 were also shown to upregulate PTEN transcription in fibroblastic cells via Egr-1 activation, thus suggesting that histone acetylation is implicated in the regulation of PTEN expression.108 Finally, recent studies have suggested that aberrant expression of microRNAs (miR-21, miR-19a, miR-17-92 and miR-214), a class of genes encoding single-stranded RNA molecules partially complementary to one or more messenger RNA molecules, affect PTEN expression.112-115 Of particular interest is miR-21, which downregulates PTEN expression in HCC and affects cell growth, migration and invasiveness of cancer cells.112
Is PTEN a therapeutic target to cure liver diseases?Intense investigations are pursued to identify pharmacological PTEN inhibitors, by both the academics and pharmaceutical companies, in particular with the aim to modulate the insulin action and alleviate insulin resistance and diabetes. In these efforts, efficient small molecule inhibitors targeting PTEN activity have been recently developed.116-118 However, accumulating data call for cautiousness in considering this strategy, in particular for liver-related diseases. Indeed, PTEN inhibitors should be potentially expected to aggravate liver disorders, such as NAFLD, viral-related metabolic dysfunctions and HCA/HCC, for which PTEN downregulation appears as an important feature with functional pathological outcomes. On the contrary, a more promising approach could be to restore physiological PTEN expression in injured hepatocytes in order to hopefully counteract liver insulin resistance, steatosis and potentially more serious disorders such as liver steatohepatitis/fibrosis and HCC. For instance, specific inhibition of IKK/NF-KB and mTOR signaling in the liver, which are exacerbated in several hepatic pathologies, and which are involved in the molecular mechanisms decreasing PTEN expression in hepatocytes,16,100 are potential therapeutical strategies thinkable to reach this goal. In this regard, mTOR and NF-KB inhibitors have been developed and are currently used in clinic or tested in pre-clinical trials.119-121 The specific targeting of such compounds to the liver remains however an issue to solve in order to avoid potential important side effects of systemic administration of these drugs. Other attracting therapeutic targets to modulate PTEN expression/activity in the liver are microRNAs, in particular miR-21, which was shown to downregulate PTEN expression in hepatocytes and to be involved in hepatocyte proliferation and invasiveness.112 In this regard, pharmaceutical companies are investing considerable financial resources to develop effective microRNAs inhibitors and/or activators to treat a variety of pathologies.122,123 Even whether specific targeting of drugs to hepatocytes can be achieved, it still remains that modulators of mTOR/NF-KB activity, or microRNA expression, are likely to affect a multitude of pathways or targets in the liver, and thus to generate important and unwanted side effects. An in depth knowledge of the role and regulation of PTEN in liver diseases, as well as additional pre-clinical and clinical trials, are thus needed to envisage therapeutic strategies modulating specifically PTEN expression and hopefully to render PTEN a site-specific «druggable» target in order to alleviate and cure human liver diseases.
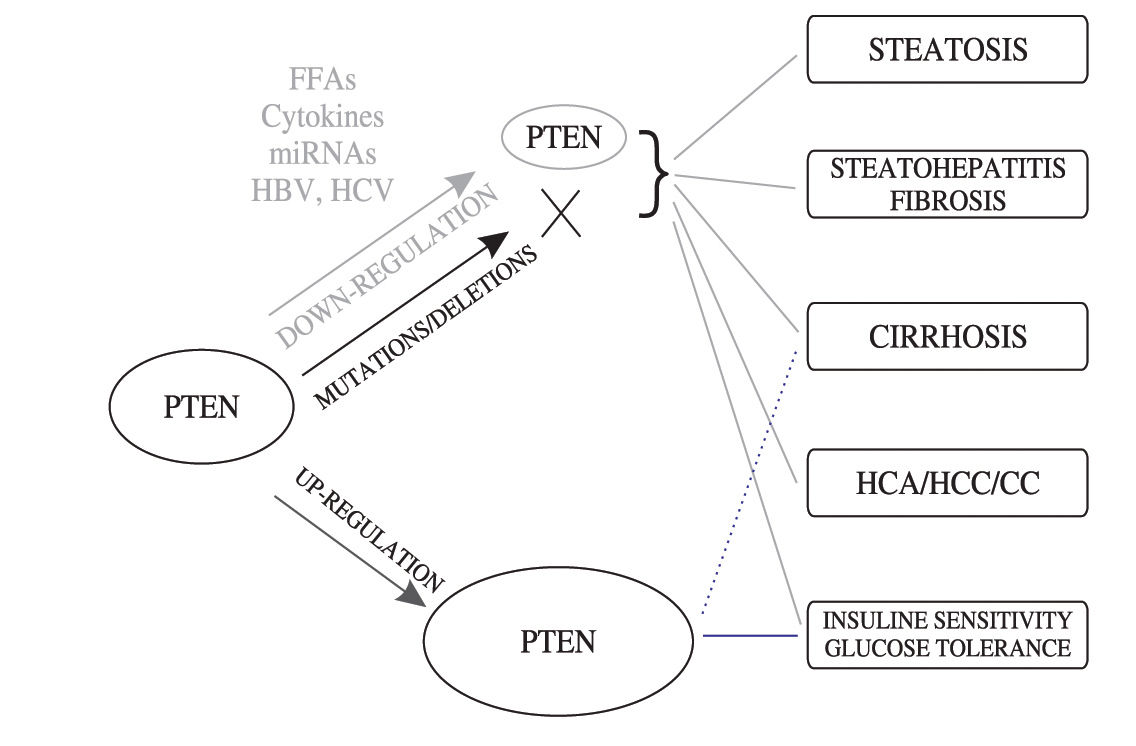
ConclusionsIn this review, we discussed the growing body of experimental and epidemiological evidence pointing out to the tumor suppressor PTEN as a major actor involved in a broad range of liver disorders, ranging from clinically benign (e.g. steatosis) to serious (e.g. HCC) hepatic pathologies (summarized in Figure 3) PTEN appears to act in the liver at the crossroad of processes controlling both metabolism and cell proliferation/apoptosis. In this regard, it is now clear that instead of mutations or deletions, dysregulations of PTEN expression or activity by a wide variety of mechanisms, including the action of metabolic factors, toxins or viral components, is likely playing a crucial role in the development and/or progression of multiple hepatic dysfunctions and uncontrolled hepatocytes proliferation. In particular in the cancer field, increasing evidence now indicates that partial loss of the PTEN expression/function, or PTEN haploinsufficiency, is sufficient to promote the development of cancer (e.g. prostate or liver cancer) thus challenging the Knudson’s «two hits» model for tumor suppressors (see also review by Salmena et al 69). In the era where genetic mutations are regarded as the almost unique causes of cancer, studies of the PTEN role in liver pathologies support an old-fashioned concept implicating a tight connection between metabolic diseases and cancer development.
Impact of alterations of PTEN expression, or PTEN mutations/deletion, in the development and progression of a wide spectrum of liver disorders. The dotted line indicates a potential involvement of ethanol-induced PTEN upregulation in the development of liver cirrhosis. Abbreviations: FFA, free fatty acids; miRNAs, microRNAs; HBV, hepatitis B virus; HCV, hepatitis C virus; HCA, hepatocellular adenomas; CC, cholangiocellular carcinomas; HCC, hepatocellular carcinomas.
This work was supported by funds of the Swiss National Science (310000-120280/1), the Reuter, The Sir Jules Thorn Charitable Overseas Trust Reg. and the Eagle Foundations awarded to MF.