Background and aims. Practitioners treating hepatitis C (HCV) provide healthcare to a special population with high rates of substance abuse and psychiatric disorders. We investigated the psychosocial profile in HCV patients and tested what variables affect commencement of antiviral therapy.
Material and methods. Recreational drug use (RDU), marijuana (THC), alcohol use, and psychiatric history were initially investigated with a questionnaire prior to history and physical. Following an educational intervention, we reinterrogated patients for RDU and THC use, and revision of initial statement was documented. Variables affecting commencement of antiviral therapy were analysed with logistic regression.
Results. Out of 153 patients, 140 (92%) answered the questionnaire. Intervention increased total yield by 6%, however, 39% (11/28) of those initially denying use revised their statement. Drug screening identified 9 more patients with RDU/THC use. Half of patients consuming alcohol were heavy drinkers, and psychiatric disease was identified in 54%. Only 73 (48%) of 139 patients eligible for antivirals received treatment. Multivariable analysis revealed that younger patients (OR = 1.04, 95% CI 1.01-1.08), and those testing positive on drug screen (OR = 0.41, 95% CI 0.19-0.92) were less likely to be treated. Denial by insurance and loss to follow-up were the most common reasons for not starting antiviral treatment.
Conclusion. Substance abuse is highly prevalent among HCV patients, and it is difficult to tell prior from current users. Integral care of HCV patients should include a diligent screen for substance abuse and rehabilitation referral, aiming to increase the pool of patients eligible for antiviral therapy. This can only be achieved through a multidisciplinary approach.
In the United States, an estimated 4.1 million people have been infected with hepatitis C virus (HCV), among whom 3.2 million are chronically infected.1 Substance abuse is a major public health problem. Recreational drug use (RDU), specifically among people who inject drugs, is disproportionately associated with HCV infection, and it is responsible for a substantial burden of the disease in the community. Intravenous drug use (IDU), is the main mode of HCV transmission in the United States:2,3 it is the main risk factor identified among patients with chronic hepatitis C, and it continues to contribute to acute HCV infections as indicated in a recent nationwide surveillance program.4 Non-injection drug use has also been associated with higher prevalence of HCV infection. Cannabis is frequently used among patients with HCV infection. Tetrahydrocannabinol (THC) exerts its biological effects via cannabinoid receptors CB1 and CB2. Although THC has been linked to more advanced stages of fibrosis, recent data suggest that CB1 and CB2 have an opposite effect on hepatic fibrogenesis and carcinogenesis,5,6 bringing uncertainty to the end result of chronic cannabis exposure.
Alcohol is another established driver of disease progression in HCV, with excessive consumption leading to acceleration of chronic liver disease.7 Concomitant HCV infection and alcohol abuse act synergistically to aggravate hepatic injury, enhance fibrosis, increase the risk of cirrhosis and progression to HCC.8 Substance abuse often occurs in the context of psychiatric comorbidity.9 There is higher prevalence of psychiatric disorders in patients with RDU and alcohol abuse,10–12 and uncontrolled psychiatric ailments are a trigger for substance abuse initiation and recidivism.13
With the introduction of directly acting antiviral (DAA) agents, treatment modalities for HCV have considerably evolved from the interferon era. DAA’s have fewer complications and drug-to-drug interactions requiring less intense monitoring and shorter treatment courses to achieve sustained virological response (SVR).14 Also, HCV treatment has proven to be safe and effective among people who inject drugs, and hence advocated by international guidelines. Media involvement in advertising the successful HCV drug-development story has attracted people with ongoing substance abuse to seek antiviral therapy with DAA. However, substance misuse may affect adherence to antiviral therapy, rate of progression to fibrosis and third-payer support for continued treatment.15–18 Therefore, efforts to eradicate HCV infection should include trained providers able to identify and provide special attention to people suffering from substance abuse and related ailments. Such strategy would increase the rate of successful antiviral therapy, minimize the burden of associated morbidities, reduce transmission and reinfection rates, and favor regression of fibrosis.19,20
The current study was undertaken to characterize the substance abuse and psychiatric profile at a specialty clinic centralizing the care of all HCV patients in a referral center. The primary goal was to investigate RDU disclosure among HCV patients before and after a brief educational intervention aiming to improve response rate. As a secondary goal, we investigated patterns of alcohol drinking, and coexisting psychiatric disease. Finally, we tested whether the information obtained from this substance abuse and psychiatric profile impacted commencement of antiviral therapy.
Material and MethodsPopulationPatients were selected from the Viral Hepatitis Clinic at the University of Arkansas for Medical Sciences. This is a tertiary referral clinic responsible for coordinating antiviral treatment for all patients with chronic viral hepatitis on campus, and the largest of its kind in central Arkansas. Consecutive new patients with chronic HCV attending the clinic between January 2014 and April 2015 were included in this study. Follow up for commencement of antiviral therapy occurred until August 2016. The Institutional Review Board of the University of Arkansas for Medical Sciences approved this study. Informed consent was not required due to interrogation strategy being part of our standard of care.
Study design and data collectionBefore their visit to the clinic, all patients were provided with a questionnaire inquiring about the use of any (Any), intravenous (IV), snorted (S), other (O) RDU, and cannabis (THC) use. During the interaction in the clinic, the provider took special time to educate patients on the impact of RDU on the transmission and clinical course of HCV, thereby justifying and emphasising the importance of disclosing an accurate substance abuse history. This brief educational intervention was followed by verbal reinterrogation on RDU and any other drug use including THC. After the clinic visit, patients were asked to provide a urine sample to test for methamphetamine, cocaine, barbiturate, opiate, benzodiazepine, phencyclidine (PCP), methadone and THC use.
In order to better characterize each patient’s psychosocial profile, the questionnaire included a detailed alcohol use history, as well as psychiatric history and use of prescribed medications. Providers took special attention in characterizing the pattern of alcohol drinking. In all cases, a retrospective chart review was performed to further investigate the psychosocial profile, and to cross reference for any missing or incomplete data. Moderate drinking was defined as up to 1 drink per day for women and up to 2 drinks per day for men, according to the Dietary Guidelines for Americans 2015-2020, U.S. Department of Health and Human Services and U.S Department of Agriculture. Heavy drinking was defined as 8 or more drinks a week for women and 15 or more drinks a week for men. The NIAAA (National Institute on Alcohol Abuse and Alcoholism) definition was used to identify binge drinking: 4 or more drinks for women and 5 or more drinks for men per drinking occasion.21
Commencement and type of antiviral therapy were documented, along with reasons for lack of start of treatment including third-payer denial (when applicable). Whenever patients received antiviral therapy, compliance and final virological response at 12 weeks (SVR12) were recorded. Fibrosis staging, whether based on biopsy reports, transient elastography, or clinically (complications from portal hypertension defining cirrhosis) was documented as well.
Statistical analysisData are presented as percentages, mean ± SD or median (interquartile range, IQR), as appropriate. Categorical variables were compared with χ2 test or McNemar’s test (before and after agreements), whereas continuous variables were compared with t-test or Mann-Whitney rank sum test. Multivariate analysis on predictors of commencement of antiviral therapy was performed by means of logistic regression. Post-regression analyses including investigation of goodness-of-fit and collinearity were performed, as appropriate. All statistical tests were implemented with Stata 12 (StataCorp, College Station, TX).
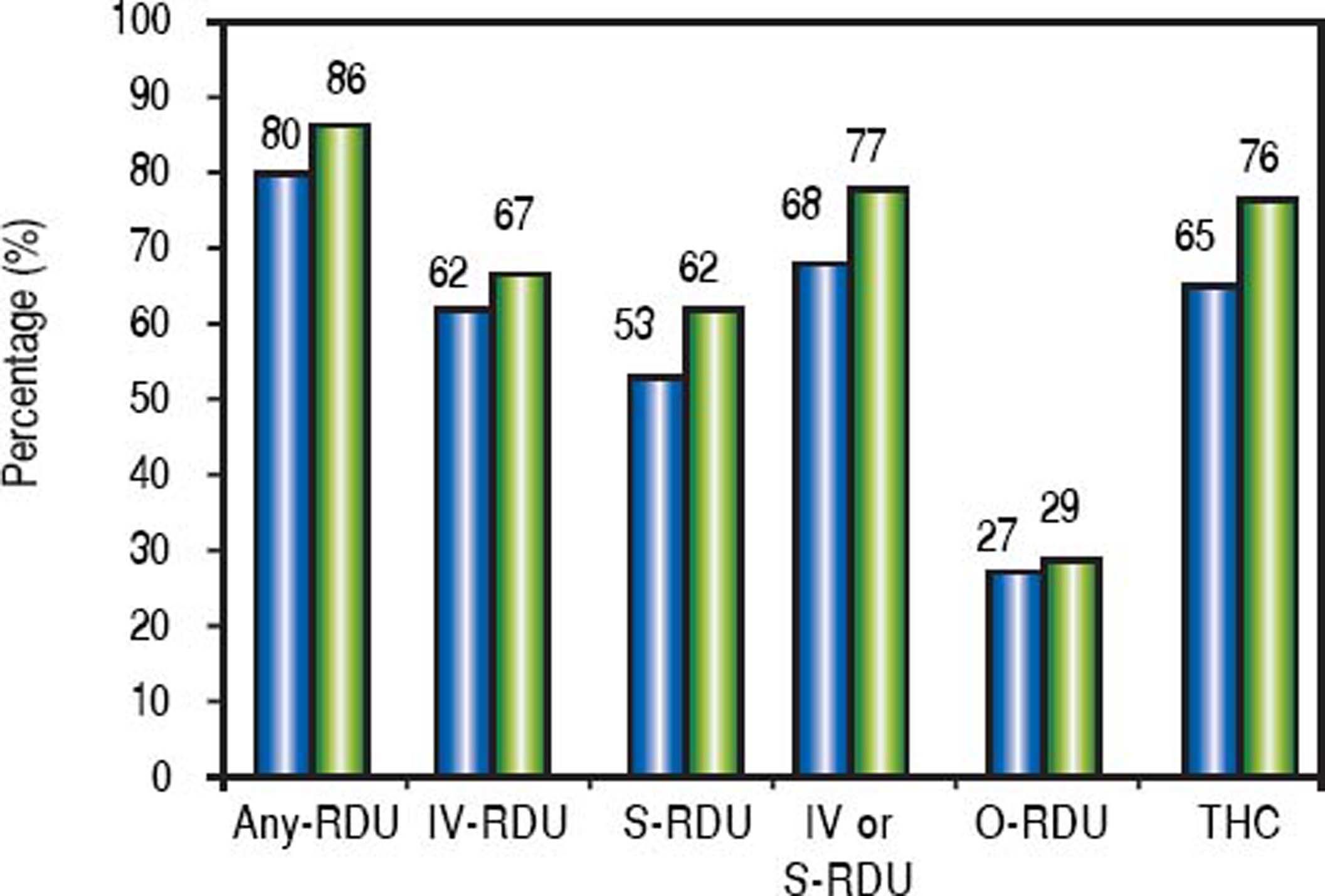
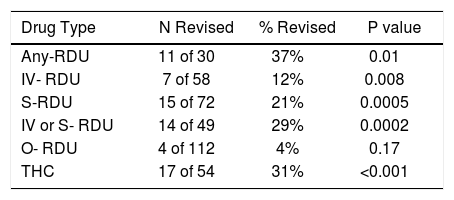
ResultsA total of 153 patients (mean age 50 ± 12 years, males 52%, females 48%) were included. One hundred and forty (92%) subjects answered the questionnaire provided to them before clinic visit, but 13 (8%) declined to fill out the substance abuse portion, in spite of asking them on a second occasion if they had anything to disclose. The history of RDU obtained before and after the educational intervention (confirmation of accuracy of the questionnaire during the clinical visit) in the whole population is shown in figure 1. Except for O-RDU, in all cases patients revised their statement revealing a significantly increased proportion of RDU and THC use. When considering only the patients that initially denied use of RDU or THC, the net gain in drug disclosure was 11/28 (39%) for Any-RDU, 7/58 (12%) for IV-RDU, 15/71 (21%) for S-RDU, 4/108 (4%) for O-RDU, and 17/54 (31%) for THC (Table 1). ORDU consisted of: methamphetamine smoking in 26 (59%), cocaine smoking in 11 (25%), use of hallucinogens (mushrooms and LSD) in 4 (9%), non-prescribed opioids/benzodiazepines in 1 (2%) and non-specified in 2 (5%). Whenever IV-RDU or S-RDU was not identified, another risk factor for HCV transmission was noted in 83% of cases (29/35), as shown in table 2.
Disclosure of RDU obtained before and after intervention. Black bars show frequency of corresponding recreational drug use (RDU) before educational intervention, whereas gray bars show frequency after intervention. IV: intravenous. S: snorted. THC: tetrahydrocannabinol. O: others.
Risk factors for HCV infection other than IV recreational drug use.
| Other Risk factors | Frequency | Percentage |
|---|---|---|
| Blood transfusion | 10 | 21% |
| Tattoos | 14 | 30% |
| Vertical / Household contacts | 8 | 17% |
| Needle stick / occupational | 3 | 6% |
| Massive air gun vaccination | 2 | <1% |
| Prison | 8 | 61% |
| High risk sexual behavior | 3 | 7% |
Urine drug screening was performed in 123 (80%) patients: 89 were tested once (58%), and 34 at least twice (22%). In 45 out of 123 patients (37%) at least one positive drug screen was documented. Methamphetamine was positive in 14 (31% - including one patient adamantly denying any drug use), cocaine in 2 (4%), and THC in 29 (64% - including 8 patients adamantly denying THC use) samples. A detailed review of prescribed or over the counter medications could not disclose possible cross-reactivity yielding false positive results to methamphetamine drug testing.
We were able to collect detailed data on alcohol use in 136 subjects. Current or prior ethanol use was reported in 118 patients (87%), of whom 38 (28%) were current drinkers. For all patients, median daily ethanol consumption was 59 g/d (23 to 137), with a mean duration of 20 (9 to 32) years. Regarding drinking patterns, 51/104 patients (49%) self-reported heavy drinking, 49/99 (49%) were daily drinkers, and 15/104 (14%) were binge drinkers. Median daily ethanol use was 88 g/d (IQR 62 to 205), and 14 g/d (IQR 5 to 24) in heavy and non-heavy drinkers, respectively (p < 0.001).
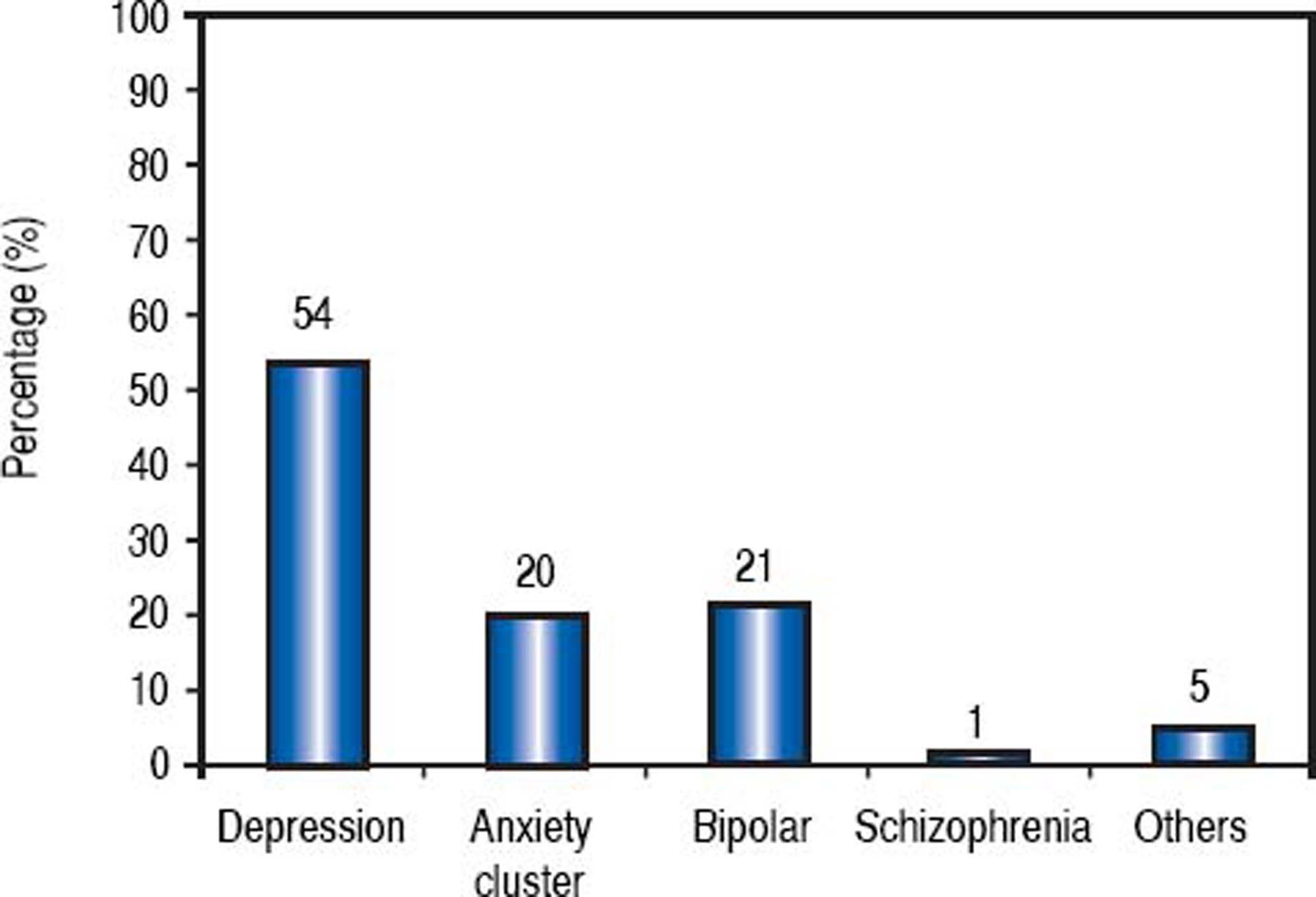
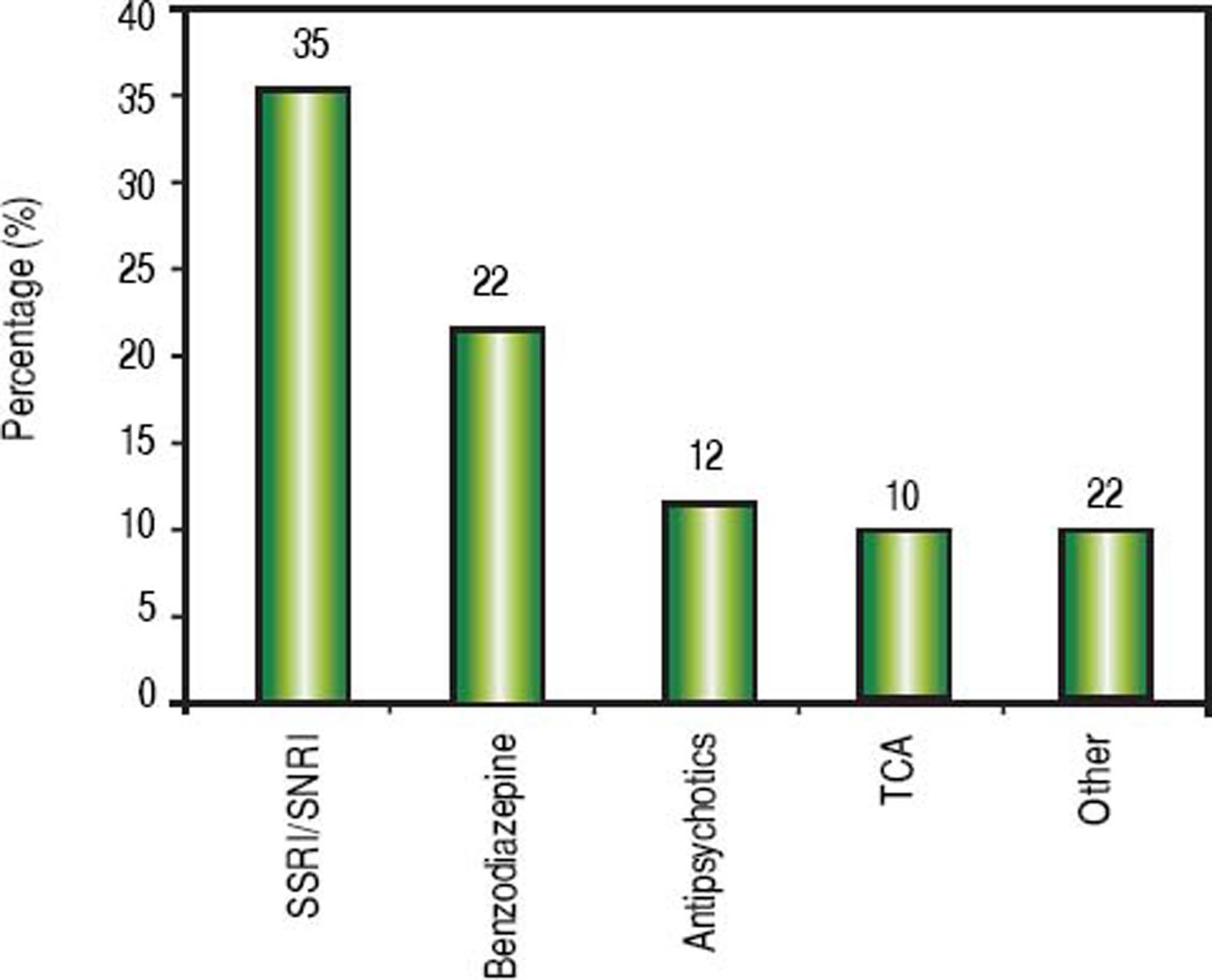
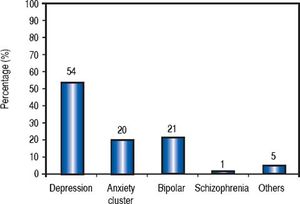
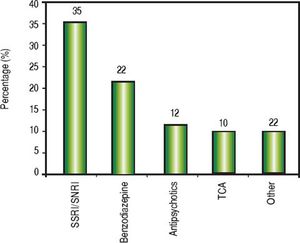
Psychiatric history was positive in 84 patients (54%), given that at least one prior or active psychiatric condition could be identified. Notably, 27 patients disclosed having 2 psychiatric diseases, and 8 reported 3 psychiatric diseases. Depression and other mood disorders were seen in almost 75% of patients bearing a psychiatric condition (Figure 2). Furthermore, 60 patients (39%) were using at least one psychiatric medication at the time of their initial evaluation: 29 patients received 2 medications and 6 patients were on 3 psychiatric drugs. A breakdown of medications identified is shown in figure 3.
During a mean follow-up of 21 ± 5 months, a total of 73 of 139 (53%) eligible patients were started on antiviral therapy. Fourteen cases (of the 153 total) were noneligible due to spontaneously resolved HCV infection or prior SVR (referred on the basis of a positive anti-HCV but found to have an undetectable HCV RNA on initial visit). These patients were eliminated from further analyses. Antiviral regimens included: ledipasvir/sofosbuvir in 42 (58%), sofosbuvir + ribavirin in 14 (19%), paritaprevir/ ombitasvir/dasabuvir ± ribavirin in 9 (12%), peginterferon + sofosbuvir + ribavirin in 5 (6%), and simeprevir + sofosbuvir ± ribavirin in 3 (4%). Fibrosis staging, available for 92 patients, was as follows: F0 in 12%, F1 in 20%, F2 in 13%, F3 in 14%, F4 in 41%. There were no differences in the rate of commencement of antiviral therapy among the different stages of fibrosis (rate of 63% to 69% across all stages; p = 0.98).
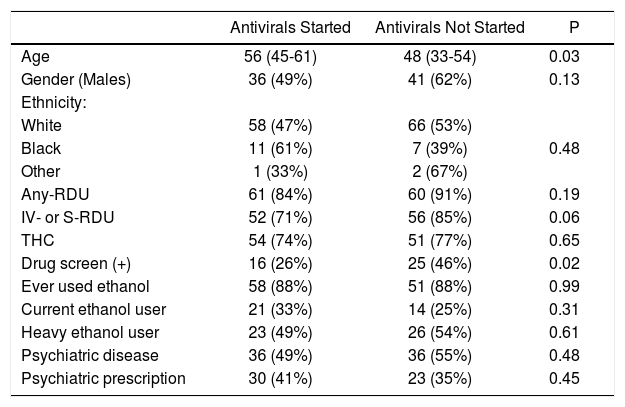
Reasons for not starting treatment were as follows: lost to follow-up in 42/66 (64%), denied by insurance or third payer in 19/66 (29%), and other reason in 5/66 (7%). Univariate analysis aiming to identify factors potentially affecting start of antiviral therapy is shown in table 3. On multivariable analysis, only age (OR = 1.04, 95% CI 1.01-1.08) and having a positive drug screen (OR = 0.41, 95% CI 0.19-0.92) were independently associated with lack of commencement of antiviral therapy. Patients testing positive in drug screen were more likely to be lost to follow-up or denied by insurance (25 [61%] with positive screen vs. 27 [36%] with a negative screen, p = 0.01). Notably, age did not affect the likelihood of being drug-screened. Sensitivity analyses adjusted for stages of fibrosis ( ≥ 2, ≥ 3, or 4) did not change the association between positive screening and commencement of antiviral therapy on multivariable models (data not shown).
Univariate analysis of factors affecting the likelihood of commencement of antiviral therapy.
| Antivirals Started | Antivirals Not Started | P | |
|---|---|---|---|
| Age | 56 (45-61) | 48 (33-54) | 0.03 |
| Gender (Males) | 36 (49%) | 41 (62%) | 0.13 |
| Ethnicity: | |||
| White | 58 (47%) | 66 (53%) | |
| Black | 11 (61%) | 7 (39%) | 0.48 |
| Other | 1 (33%) | 2 (67%) | |
| Any-RDU | 61 (84%) | 60 (91%) | 0.19 |
| IV- or S-RDU | 52 (71%) | 56 (85%) | 0.06 |
| THC | 54 (74%) | 51 (77%) | 0.65 |
| Drug screen (+) | 16 (26%) | 25 (46%) | 0.02 |
| Ever used ethanol | 58 (88%) | 51 (88%) | 0.99 |
| Current ethanol user | 21 (33%) | 14 (25%) | 0.31 |
| Heavy ethanol user | 23 (49%) | 26 (54%) | 0.61 |
| Psychiatric disease | 36 (49%) | 36 (55%) | 0.48 |
| Psychiatric prescription | 30 (41%) | 23 (35%) | 0.45 |
Noncompliance with antiviral regimen was identified in 10/73 treated patients. Only in one instance, however, was noncompliance considered significant (lapse of 2 weeks off antiviral therapy), and remaining cases reported missing no more than 3 doses. Among 58 patients with available information on final outcome of antiviral therapy, SVR12 was achieved in 57 (98%). Data was underpowered to analyse the effect of psychosocial profile on antiviral compliance or achievement of an SVR12.
DiscussionThe impact of collecting accurate and relevant history as a key step in managing gastrointestinal diseases has been the focus of recent discussions.22 The results of our study demonstrate that even with routine history taking including use of questionnaires, reconciliation of patient-provided data remains inaccurate for substance abuse. However dedicating clinic time to educate, thoroughly interrogate and evoke history of RDU or THC use can increase yield by 6% to 30%. Moreover, when considering only patients providing a negative response, we found that up to 39% revised their statement accepting RDU. Although this systematic approach increased the yield of positive responses, it was still insufficient as a few cases with positive drug testing were identified in the absence of statement revision.
An increasing trend in intravenous drug injection in the United States has been observed in the last decade.4 This mainly involved injection of prescription opioids among persons who initially used oral opioid analgesics.23,24 Importantly, this increase in IDU overlapped with outbreaks of HCV and HIV infection, what has been less evident among areas where targeted risk reduction efforts like syringe service programs were incorporated.25 There is growing evidence on the health-related impact of non-injection drug (NIDU) use paraphernalia through sniffing, snorting or smoking drugs such as cocaine, heroin or methamphetamine, including higher risk of non-parenteral HCV transmission.26–29 Crack smokers are prone to oral lesions (burns, blisters, and sores) that facilitate the oral transmission of blood-borne infections.28 Also, high-risk sexual behavior, including exchanging sex for drugs or money, multiple sex partners and unprotected encounters are often associated with intensive crack use.26 A systematic review found HCV prevalence rates among people with non-injectable recreational drug use to be 2-35% (median of 14%), what is significantly higher than the general population.30 Collectively, these findings call attention to better identification of these vulnerable groups and creation of interventions to minimise risky behaviors and prevent HCV transmission.
Although high rates of HCV reinfection were observed among people who inject drugs in the past recent studies indicate that HCV reinfection rate remains relatively low after antiviral therapy and that there is little benefit in withholding HCV treatment due to concerns of reinfection alone.31 This is particularly true with the potent, newer, DAA’s that provide safe, effective and tolerable therapy counterbalancing reinfection rates. New DAA’s are pharmacologically compatible with methadone substitution therapy and other de-addiction strategies. Moreover, the full impact of DAA’s on HCV epidemiology will be realized only after all HCV-infected individuals are engaged in antiviral treatment,32 aiming to eradicate HCV from the community. Therefore, these patients should be identified, not for exclusion from antiviral therapy, but to counsel them on the consequences of risky behavior and the benefits of safer practices and link them to local addiction rehabilitation facilities or support programs facilitating supervised drug consumption associated with a lower reinfection rates.
Alcohol is considered to be the most used and abused psychoactive drug worldwide.33 In agreement with this statement, more than 85% of our study population reported alcohol use, of whom nearly half had harmful/hazardous alcohol use. Notably, 28% of patients were current drinkers. Alcohol abuse also contributes to risky behavior patterns34,35 and is associated with higher incidence36 and prevalence of HCV infection compared to the general population.37 It is well known that alcohol plays a major role on liver disease. Recently, even moderate alcohol consumption was linked to cirrhosis in a nationwide Danish study.38 Marijuana is another widely used recreational drug and almost 80% of our patients were either past or current THC consumers. The role of cannabinoids in cirrhosis is a widely debated topic. There is experimental evidence linking CB1 overexpression to increased steatosis,39 whereas, THC inhibits alcohol-induced inflammation and steatosis via CB2,40 in addition to suppressing proliferation and inducing apoptosis of hepatic myofibroblasts resulting in an antifibrotic and hepatoprotective effect.41 Although a retrospective study indicated daily THC use independently predicted fibrosis progression,42 more evidence is needed to clarify final effects of smoking marijuana on hepatic fibrogenesis. In what respects to regression of fibrosis, alcohol use in HCV-related cirrhosis would negate patients from the full benefits of viral eradication by perpetuating the fibrogenic process.43 To date, there is no data on the mechanism of THC in fibrosis regression after viral eradication. Whereas it is crucial to identify HCV patients with alcohol use disorder and refer them to effective de-addiction and rehabilitation programs to reduce future liver damage, the same cannot be said about THC yet, but arguably evidence points in the same direction.
Treatment regimens for HCV infection require commitment on the part of the patient. RDU, alcohol abuse and psychiatric disease are often interrelated, with one leading to the other in many cases.44,45 Since uncontrolled mental illness can affect treatment adherence and herald RDU and alcohol relapse among people with prior history, obtaining a precise history of psychiatric disorders is just as critical as acquiring accurate substance abuse and alcohol history. Also, mental illness can be exacerbated during antiviral therapy, and psychiatric side effects are still seen in the DAA’s era (even though these were not different from placebo in registration trials). Finally, drug-to-drug interactions between psychotropic drugs and DAA’s can be better managed when psychiatric disease is better understood and there is proper provider support. Our study revealed that 50% or more of the subjects had at least one psychiatric condition; depression being the most common disorder, and almost 40% reported using at least one psychiatric medication. Importantly, we did not observe compliance issues with psychiatric disease. SSRI’s were the most commonly used medications. Nearly 35% patients were either on an SSRI or SNRI, and more than 40% on benzodiazepines (BDZ), tricyclic antidepressants (TCA) or antipsychotics. In general, SSRI and SNRI have a broad therapeutic index, while BDZ, TCA and antipsychotics have a higher potential for drug-to-drug interactions.46,47 This is relevant for providers prescribing DAA’s, as adjustments on psychotropic drugs are potentially expected in over a third of HCV patients looking for antiviral therapy.
During the timeframe of the study, almost 50% of our patients could not be commenced on antiviral therapy. Multivariable analysis revealed that younger patients and those tested positive on drug screen (RDU or THC) were less likely to be treated. Denial by insurance and loss to follow-up were more frequently seen in those with positive drug screen. This included some patients providing false contact information whom could not be traced following the index clinic visit. In contrast, prior or current alcohol abuse or psychiatric illness, did not affect commencement of antivirals. Access to DAA’s has been a matter of intense debate given the exorbitant costs. In response, third-party payers went from universal treatment (during the peg-interferon and ribavirin era) to approving DAA’s only for patients with advanced fibrosis or cirrhosis - although, this varies across insurance plans and geographic regions. We have recently noted most third-party payers becoming extremely sensitive to substance abuse and psychosocial issues, using them to substantiate DAA denial on the basis of daunting compliance. This includes testing positive only to THC, what lacks a clear physiologic rationale, particularly at a time of growing approval and legalisation of THC use. We recommend addressing substance abuse and psychiatric disease in parallel, while aiming to prescribe DAA, given the irrefutable evidence favoring treatment of these patients. Furthermore, universal therapy has been demonstrated to be the most effective strategy for reduction of HCV-associated cirrhosis, liver complications, and death.48
Our study had some limitations. Urine screens were not performed following special regulations (i.e. monitored collection and secured water sources), and on some occasions we were able to identify that patients had provided us with a fluid different from urine (likely water given the lack of specific gravity). Not all patients were drug tested, and on occasions orders were cancelled by the provider following a direct request from patient when the latter revealed that results would turn out positive - with the intention of building up rapport with active substance users. Finally, regarding alcohol use, we did not use phophatidylethanol testing to objectively document chronic heavy drinking.
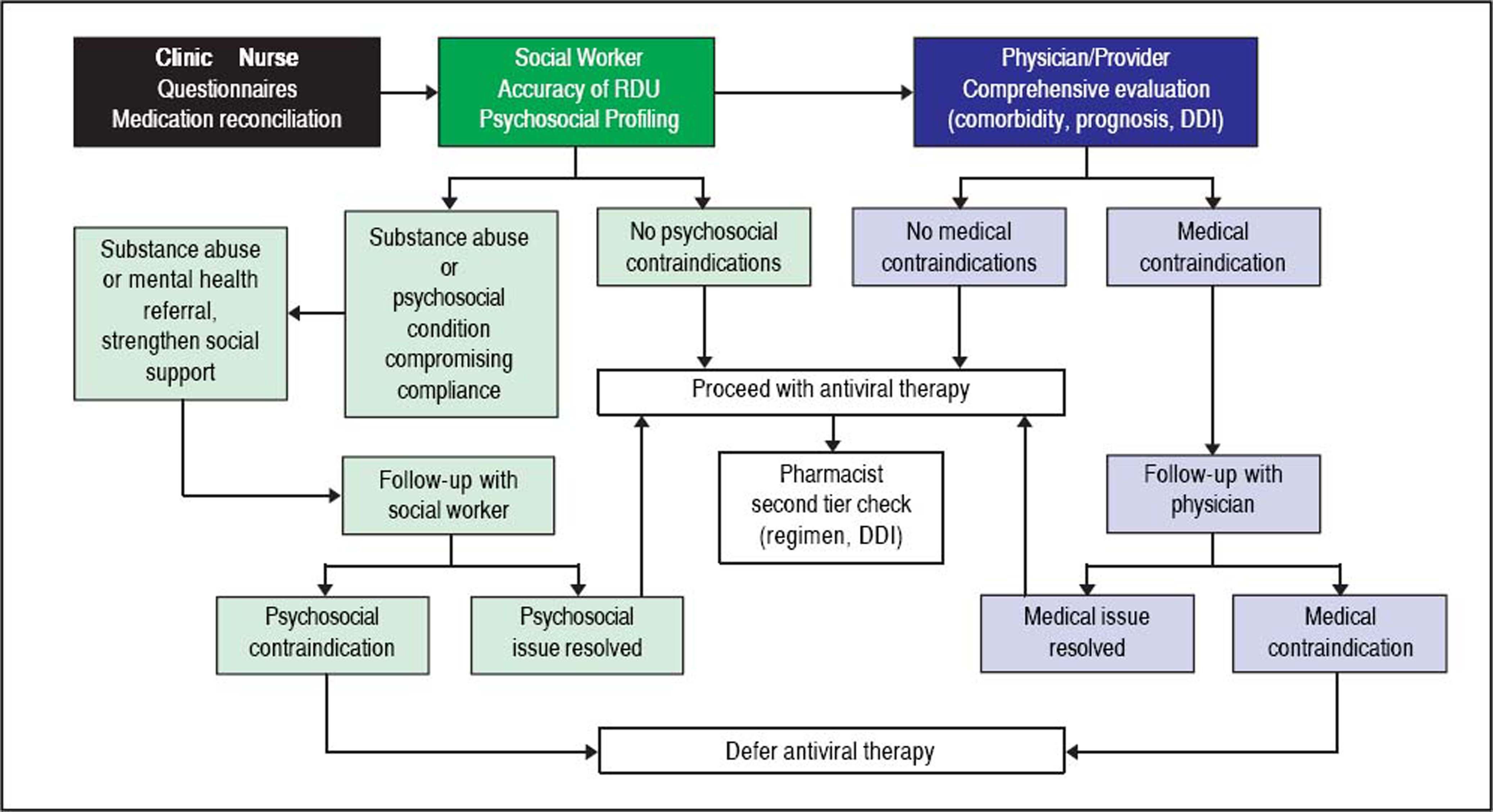
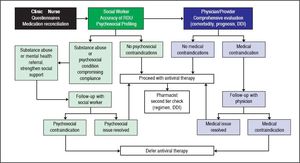
Given the intricate relationship between substance abuse, psychiatric disease and HCV, and its impact on approval for antiviral therapy, we believe that obtaining an accurate psychosocial history is critical before planning antiviral therapy. In figure 4 we propose a strategy based on a multidisciplinary team for the evaluation of patients attending specialized clinics caring for HCV patients. This strategy would permit the identification of patients with ongoing substance abuse issues in order to assess readiness for stopping drug/alcohol use or willingness to undergo a rehabilitation program, an in-depth discussion on available resources to avoid reinfection if antiviral treatment concomitant to ongoing drug use is planned, individualization of follow up to assure compliance with medication and of monitoring for relapse or reinfection, and counselling on the risks associated with ongoing substance abuse. A randomized clinical trial recently confirmed that an integrated HCV care strategy including a midlevel mental health provider improved commencement of antiviral therapy and SVR rates among patients with an unfavorable psychosocial profile.49 Moreover, a recent publication found drug-related incidents as the main cause of death among patients younger than fifty that had achieved viral eradication, whereas hepatic causes (mainly liver cancer) were the main drivers of mortality in those older than fifty.50 Clinics caring for HCV patients should therefore provide the proper psychosocial support as such strategy would not only improve liver outcomes, but overall mortality, particularly in people younger than fifty.
Advocating client confidentiality, careful history taking and reconciliation in a non-judgmental empathetic environment, and treating patients with respect can make a huge difference in building trust and long-term patientprovider relationships. Responsible and thorough substance abuse evaluation in concert with effective proven interventions that maximize abstinence and minimize behaviors with risk for reinfection might finally convince all third-party payers to provide universal care to all HCV-infected individuals. In the end, all human beings, particularly those taking the right steps to take good care of themselves, deserve antiviral therapy, irrespective of the hurdles that health care systems currently impose worldwide.
Abbreviations- •
DAA: directly acting antivirals.
- •
HCV: hepatitis C virus.
- •
IDU: injection drug use.
- •
IV: intravenous.
- •
NIAAA: National Institute on Alcohol Abuse and Alcoholism.
- •
NIDU: non-injection drug use.
- •
O: other.
- •
PCP: phencyclidine.
- •
RDU: Recreational drug use.
- •
S: snorted.
- •
SNRI: selective serotonin norepinephrine reuptake inhibitors.
- •
SSRI: selective serotonin reuptake inhibitors.
- •
SVR: sustained viral response.
- •
TCA: tricyclic antidepressants.
- •
THC: tetrahydrocannabinol.
Authors would like to thank Arun Chowdary, MD for his help during preparation of this manuscript.
DisclosureAndres Duarte-Rojo.
Consultancy: Gilead Sciences.
Research Grants: Gilead Sciences, Vital Therapies, Ocera Therapeutics.
Financial SupportAndres Duarte-Rojo receives support from the University of Arkansas for Medical Sciences College of Medicine Clinician Scientist Program.