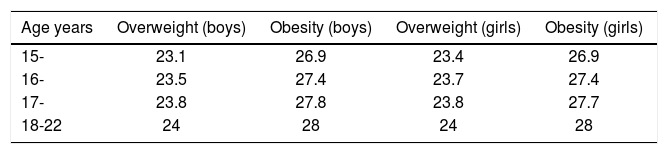Introduction and aim. To investigate the association of adolescent obesity with nonalcoholic fatty liver disease (NAFLD) and related risk factors in Xi ’an, China.
Material and methods. A total of 4141 adolescents (2,061 girls and 2,080 boys, mean age: 18.62 ± 0.66 years, age range 15-22 years) were enrolled in this investigation. Anthropometric index was measured, and liver ultrasonography and liver function biochemical test were performed in all the subjects. T test, χ2 test and logistic regression analysis were used for statistical analyses. Results. The total rates of obesity was 7.9% (08/4,141). The prevalence rate of NAFLD was 8.1% (335/4141) with a declining trend from obesity, overweight to normal BMI. NAFLD prevalence rate in obese boys was significantly higher than in girls (χ2 = 56.5, P < 0.01). BMI, body weight, ALT, and AST in NAFLD group were higher than in non-NAFLD group (P < 0.05). The tangent point of ALT was 36 U/L using Youden index in boys, and 33 U/L in girls.
Conclusion. The prevalence of obesity and NAFLD in adolescents is higher in Xi’an than anticipated. Body weight and BMI may be the associated independent risk factors of NAFLD.
Non-alcoholic fatty liver disease (NAFLD) includes nonalcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH). NAFLD has become the main cause of chronic liver diseases in children and adolescents with an increasing trend of obesity and related metabolic syndrome worldwide.1 The increased incidence of NAFLD suggests the higher number of obese people.2 An Italian national health and nutrition survey showed that 17% of the children from western countries were overweight, and 70-80% of the overweight children had NAFLD in 2012.3 The prevalence of overweight and obesity in high school students was 16.3% and 8.5%, respectively, and the prevalence of NAFLD was 74% in obese individuals in Shanghai in 2009,4 while the prevalence of overweight and obesity in high school students was only 13.7% and 3.9% in Shanghai in 2001. Research about child and adolescent obesity and NAFLD has drawn much attention from both medical community and the society. Chang Ming’s epidemiological survey about overweight and obese children and adolescents in Xi ’an city in 2009 showed that incidence of child and adolescent obesity was 4.67%, being 4.92% in boys and 4.40% in girls.5 However, there is no epidemiological survey about the adolescent obesity and NAFLD in Xi ’an city, China. This study aims to know the epidemic status of adolescent obesity, NAFLD and related risk factors, to find teenager high-risk groups of NAFLD, and to provide a scientific basis for early intervention of NAFLD.
Material and MethodsSubjectsA total of 4141 adolescents (2,061 girls and 2,080 boys, mean age: 18.62 ± 0.66 years, age range: 15-22 years) were randomly recruited from teenagers who took college entrance examination in Xi ’an Medical College from March to April, 2012. Exclusion criteria were hepatic virus infections (hepatitis A, B, C, cytomegalovirus and Epstein-Barr virus infections), alcohol consumption, history of parenteral nutrition, and drug-induced hepatitis or autoimmune liver disease. Subjects were asked to record the daily sleep duration, exercise duration, and seating duration for 1 months.
Examinations- •
Anthropometric index. Height was measured to the nearest 0.5 cm, without shoes, back against the wall, eyes looking straight ahead, with a right-angled triangle resting on the scalp and against the wall. Weight measurements were done with the subjects in the postabsorptive state, with an empty bladder, without shoes and in light undergarments, using a lever scale, sensitive to 100 g. The body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters squared).
- •
Liver ultrasonography. Trained operators who were blinded to all clinical and laboratory characteristics of the participants performed liver ultrasonography. Scans were performed in all subjects using a GE Vivid 7 (GE Company, USA) machine. In order to reduce interference of the gastrointestinal contents, subjects were fasting for 8h overnight. Liver ultrasonography was performed in quiet environment when the light was moderate.
- •
Laboratory tests. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were determined between 08.00 and 10.00 am after fasting for 12h overnight. Serum parameters, including ALT and AST, were measured (Olympus, AU640, Japan).
Compared with adolescents with the same BMI in the United States and Europe, Asians had higher body fat content. The BMI standards of WHO, IOTF or CDC of the United States, might underestimate the rate of obesity in Chinese adolescents. The BMI classification standards of overweight and obesity in Chinese school-age children and adolescents by the Working Group on Obesity in China6 was used (Table 1). Ultrasonic NAFLD hierarchical diagnostic standards7 were followed as shown in table 2.
Body mass index (BMI) classification standards (The BMI classification standards of overweight and obesity in Chinese school-age children and adolescents by the Working Group on Obesity in China6).
| Age years | Overweight (boys) | Obesity (boys) | Overweight (girls) | Obesity (girls) |
|---|---|---|---|---|
| 15- | 23.1 | 26.9 | 23.4 | 26.9 |
| 16- | 23.5 | 27.4 | 23.7 | 27.4 |
| 17- | 23.8 | 27.8 | 23.8 | 27.7 |
| 18-22 | 24 | 28 | 24 | 28 |
Ultrasonic NAFLD hierarchical diagnostic standards.
| Mild | Moderate | Severe | |
|---|---|---|---|
| Liver shape | Normal liver boundaries, sharp angle | Bigger liver boundaries, dull angle | Liver boundaries obviously increased, obtuse angle |
| Dot echoes on the liver parenchyma | Bulky density, enhanced echo | Bulky intensive density, enhanced echo | Thick, dense, enhancement, convex lens |
| Liver rear echo attenuation | None | Mild | Obvious |
| Intrahepatic vessels | Normal | Diminution | Squash |
| CDFI | Intrahepatic blood color: slightly dim | Intrahepatic blood color: dim | Intrahepatic blood color: dim; vessel arrangement: discontinuous |
| PV trunk diameter (cm) | < 1.3 ± 0.05 | ≤ 1.3 ± 0.05 | ≥ 1.4 ± 0.05 |
| PV blood flow velocity (cm/s) | 12.0 ± 0.05 | 11.0 ± 0.06 | 10.3 ± 0.0 |
| Spleen | Normal | Thickness of the spleen: 3.8-4.1cm | Thickness of the spleen > 4.5 cm volume increased |
CDFI: Color doplor flow Image, PV: Hepatic portal vein.
The SPSS version 15 (SPSS, Chicago, IL, USA) was used for statistical analyses. Comparisons of prevalence rates of obesity, overweight and NAFLD in different groups were performed using χ2 test. T test was applied to analyze the comparisons of BMI, body weight, ALT and AST in different groups. Logistic regression was used for multivariate analysis. A probability value of < 0.05 was considered to be statistically significant.
ResultsPrevalence rate of obesity and overweightThe total rate of obesity was 7.9% (308/4141). The prevalence rates of obesity and overweight were 9.8% (204/ 2,080) and 21.8% (453/2,080) in boys, while 6.0% (124/ 2,061) and 11.7% 242/2,061 in girls, respectively. Comparisons of prevalence rate of obesity and overweight between boys and girls were statistically significant (χ2 = 20.4, P < 0.01, χ2 = 49.5, P < 0.01, Figure 1).
Comparison of prevalence rates of obesity, overweight and NAFLD between boys and girls. * Compared with the prevalence rate of overweight in girls, P < 0.01. ** Compared with the prevalence rate of obesity in girls, P < 0.01. *** Compared with the prevalence rate of NAFLD in girls, P < 0.01.
The prevalence rate of NAFLD was 13.4% (278/2080) in boys, which was significantly higher than in girls (2.8%, 57/2061), (χ2 = 156.4, P < 0.01, Figure 1).
The NAFLD prevalence rate of the boys in normal weight, overweight and obesity groups were 1.1% (16/ 1,423), 25.6% (116/453) and 71.5% (146/204), respectively. The NAFLD prevalence rate in the girls in normal weight, overweight and obesity groups were 0.4% (6/ 1,695), 6.2% (15/242) and 29.0% (36/124), respectively. Among the different BMI groups in boys and girls, the prevalence rate of mild NAFLD was the highest, followed by the moderate, and the severe (Table 3. NAFLD prevalence rate had an declining trend from obesity, overweight to normal BMI. The NAFLD prevalence rate in obese boys was significantly higher than in girls (Figure 2, χ2 = 56.5, P < 0.01).
Comparisons of prevalence rate of US-detected NAFLD in boys and girls with different BMI.
| BMI groups | n | Mild | Moderate | Severe | Summation |
|---|---|---|---|---|---|
| Normal (boys) | 1,423 | 16 (1.6) | 0 (0) | 0 (0) | 16 (1.1) |
| Overweight (boys) | 453 | 94 (20.7) | 22 (4.9) | 0 (0) | 116 (25.6) |
| Obesity (boys) | 204 | 112 (54.9) | 28 (13.7) | 6 (2.9) | 146 (71.5) |
| Normal (girls) | 1,695 | 6 (0.4) | 0 (0) | 0 (0) | 6 (0.4) |
| Overweight (girls) | 242 | 12 (5.0) | 3 (1.2) | 0 (0) | 15 (6.2) |
| Obese (girls) | 124 | 27 (21.8) | 8 (6.4) | 1 (0.8) | 36 (29.0) |
BMI, body weight, ALT and AST in NAFLD group were all higher than those in non-NAFLD group (P < 0.05). With regard to height in both boys and girls, there was no significant difference between NAFLD and non-NAFLD groups (Table 4).
Comparison of physical development indicators and transaminase in NAFLD and non-NAFLD boys and girls, × ± s.
| Group | n | BMI | Height (cm) | Body weight (kg) | Body weight (kg) | ALT (U/L) |
|---|---|---|---|---|---|---|
| NAFLD groups(boys) | 278 | 27.6 ± 3.9 | 170.3 ± 3.6 | 70.6 ± 11.1 | 40.8 ± 23.7 | 72.3 ± 30.4 |
| Non-NAFLD groups(boys) | 1,802 | 23.3 ± 3.4 | 173.8 ± 4.5 | 60.1 ± 13.6 | 24.7 ± 9.6 | 29.6 ± 16.4 |
| t | - | 3.340 | 1.514 | 2.839 | 2.127 | 2.345 |
| P | - | 0.001 | 0.16 | 0.005 | 0.03 | 0.02 |
| NAFLD groups(girls) | 57 | 25.5 ± 2.8 | 160.2 ± 3.1 | 63.5 ± 8.9 | 35.7 ± 17.2 | 42.6 ± 21.8 |
| Non-NAFLD groups(girls) | 2,004 | 21.5 ± 2.5 | 162.0 ± 4.3 | 55.8 ± 7.1 | 18.6 ± 9.3 | 16.9 ± 6.6 |
| t | - | 3.300 | 1.237 | 2.813 | 2.601 | 2.330 |
| P | - | 0.001 | 0.35 | 0.005 | 0.01 | 0.02 |
BMI, body weight, ALT, and AST were analyzed by Logistic analysis. The results showed that the body weight and BMI may be independent risk factors of for NAFLD (Table 5).
Logistic regression analysis of NAFLD risk factors.
| Risk factors | B | SE | Wald | df | Sig | Exp (B) | 95%CI |
|---|---|---|---|---|---|---|---|
| Body weight | 2.167 | 1.036 | 4.331 | 1 | 0.027 | 9.325 | (46.056,80.944) |
| BMI | 0.381 | 0.198 | 6.132 | 1 | 0.010 | 1.467 | (19.512,30.988) |
| ALT | 1.410 | 0.913 | 2.380 | 3 | 0.123 | 0.244 | (17.120,64.522) |
| AST | 0.794 | 0.695 | 1.318 | 3 | 0.253 | 0.452 | (13.245,102.763) |
ALT numerical values were test variables, and patients with or without NAFLD were outcome variables for ROC curve analysis. Boys’ AUC was 0.912 (95% CI 0.872 - 0.941), and the girls’ was 0.932 (95% CI 0.924 -0.943), P < 0.05. The tangent point of ALT was 36 U/L using Youden index, with a sensitivity of 83.1%, and specificity of 84.5% in boys. The tangent point of ALT was 36 U/L, the sensitivity was 91.2% and specificity was 87.9% in girls (Figure 3). The total number of boys with ALT ≥ 36 U/L and the girls with ALT ≥ 33 U/L was 293, with a detection rate of NAFLD of 79.3%, accounting for 87.5% of all patients with NAFLD.
DiscussionObesity was on the WHO classification of diseases list as a kind of chronic metabolic disease caused by multiple factors as early as in 1948(ICD code E66). Overweight and obesity may lead to serious health consequences, and these risks are on the rise with a higher BMI. Chronic diseases associated with increased BMI included: cardiac-cerebral vascular diseases (including coronary heart disease and stroke), diabetes, osteoarthritis, certain cancers, such as endometrial cancer, breast cancer, and colon cancer.8
The prevalence rates of overweight and obesity were 28.1% and 9.8% respectively among China’s urban residents in 2002, and these numbers have increased to 32.4% and 13.2% respectively between 2010 and 2011. At the same time, overweight and obesity of children and adolescents became prominent in China. The overweight obesity rates of city boys and girls, and rural boys and girls are were 1.3% and 1.6%, and 0.5% and 0.6%, respectively in China in 1985. These numbers have been rising over the past 20 years, and reached to 23.2% and 13.8%, and 12.7% and 8.6% in 2010.9 In the present study, the prevalence rates of obesity and overweight were 9.8% and 21.8% in boys, while 6.0% and 11.7% in girls, respectively, which are close to those in 2010. The increased adolescent overweight and obesity rates were associated with calorie accumulation caused by excessive intake of refined food and high-sugar drinks, and sedentary lifestyle. Diet habit, physical activity facilities, and sports instruction might also play a considerable role in adolescent obesity.10 In addition, studies have shown that the risk for obesity is nearly three times higher for children who sleep less than eight hours per night. Shortened sleep patterns have also led to an increase in ghrelin and a decrease in leptin.11 The subjects of this study were senior high school students in third grade, who keep sedentary for more than eight hours a day on average, lacking physical exercise and sleep (less than eight hours daily). They were at high risk of the obesity.
With increasing teenager obesity, the prevalence of NAFLD was also on the rise. Lawlor12 reported a prevalence of NAFLD of 2.5% in British teenagers. Kang RT’s study showed that prevalence rate of NAFLD was 38.5% in children.13 Our study showed that adolescent NAFLD prevalence was 8.1% in the Xi ’an region, China. The difference between studies may be related to age range, area variation in developmental level, diet and lifestyle. Increasing incidence of NAFLD showed a trend of rapid, early onset in China. There were no obvious symptoms and signs of mild fatty liver, so the detection rate was the highest among the normal adolescents. Therefore, the early diagnosis of NAFLD was particularly important. Without early intervention, damage may occur to both the physical and mental health, leading to a decline in population quality and social comprehensive strength.
Studies have shown that obese adolescents with NAFLD are common. The American national health and nutrition survey showed that 6% of overweight and 10% of obese adolescents had NAFLD in 2011.14 Long DB’s research has shown that the detection rate of NAFLD boys is 54.7%, and the girls’ is 35.6% in obese children and adolescents.15 The prevalence rate of NAFLD was 13.4% in boys, and 2.8% in girls in our investigation, which was close to Long DB’s results. One of the reasons for prevalence difference might be the sex hormone difference between male and female. Studies have shown that estrogen may offer some protection against NAFLD.16 Another reason was that boys and girls had different dietary patterns. Boys were more likely to choose foods high in fat and calories.
Single factor analysis revealed that BMI, body weight, ALT and AST in NAFLD group were all higher than those in non-NAFLD group both in boys and girls. It was suggested that subjects in NAFLD group had overweight, obesity or abnormal hepatic function. Logistic regression analysis indicated that body weight and BMI may be independent risk factors of NAFLD. With body weight and BMI increasing, the incidence of NAFLD rises subsequently. It has been shown that the prevalence of NAFLD was closely related to obesity. BMI was a strong indicator for the youth body fat distribution; it could reflect the abdominal visceral fat and subcutaneous fat, relative to weight.17 Hagström’s study showed that a high BMI in late adolescence in men predict development of severe liver disease later in life, corresponding to a 64% increased risk for overweight men compared to men of low normal weight, or a 5% increased risk per 1 kg/m2 increase in BMI.18 According to NAFLD recommendations by the nonalcoholic fatty liver disease diagnosis and treatment guidelines of Chinese Medical Association (CMA) in 2010,19 change of lifestyle and weight control were preferred protocol for NAFLD. Clinicians, parents and teachers should develop intensive life style intervention measures suitable for teenagers, such as taking healthy diet, avoiding high quantity of calorie, fat and sugar food intake, and ensuring sufficient protein intake; low-intensity aerobic exercise is necessary for at least half an hour at a time, 2-3 times per week. Heart rate increase should be less than 50-60% of the individual basic heart rate, and weight loss should not be more than 0.5 kg per week.
Serum ALT activity, the variable most commonly measured to assess hepatic disease, fails to identify many patients with hepatic injury. Current standards for “normal” ALT level were defined by using populations that included persons with sub-clinical liver disease. Prati’s research showed that serum ALT activity independently related to BMI and to laboratory indicators of abnormal lipid or carbohydrate metabolism. Updated upper limits (for men, 30U/L; for women 19 U/L) were lower than current limits (for men, 40U/L; for women 30 U/L).20 In our study, ALT numerical values were test variables, and patients with or without NAFLD were outcome variables for ROC curve analysis in our study. The size of the AUC represented the diagnosis efficiency; the higher value showed the greater value of the diagnosis system in ROC curve analysis. When the AUC was proximal to 0.5, it lost the significance of clinical diagnosis. Diagnostic accuracy was low when AUC < 0.7, moderate when AUC was between 0.7 and 0.9, and high when AUC > 0.9. The AUC of boys and girls were 0.912 and 0.932, respectively. It was suggested that ALT is a good indicator for diagnosis of NAFLD, which was 36 U/L and 33 U/L in boys and girls, respectively. Siddiqui21 confirmed that serum ALT elevation (including normal high value range) was associated with a variety of cardiovascular disease markers (including lipid, lipoprotein, apolipoprotein, etc.) in asymptomatic individuals. The relationship among ALT, NAFLD and the risk of cardiovascular disease and mechanism need to be verified in multi-center prospective studies with large samples.
It has been known that NAFLD incidence is related to insulin resistance, genetic susceptibility, nutrition surplus, obesity, diabetes, hyperlipidemia and other related metabolic syndrome. Because the subjects of the present study were teenagers attending the university entrance exam, we were unable to analyze insulin resistance, hyperlipidemia and other risk factors. We hope to further study the NAFLD and other risk factors in the future based on the current research.
Teenagers in the growth and development period need much more nutrient intake. If balanced diet, and reasonable and persistent physical exercise were grossly under-emphasized, obesity and NAFLD would be prevalent. Teenager NAFLD is pathologically reversible; early detection and positive life behavior intervention can improve or reverse the clinical and subclinical symptoms. Therefore, reasonable lifestyle, weight and BMI control and ALT monitoring are important measures to prevent NAFLD and the premise of the overall treatment for obese adolescents.
Author ContributionsYan R designed the research and drafted the manuscript; Niu CY and Zhao HX extracted and analyzed the data; Yu L, Wang W and Tian Y collected and analyzed data; all authors have read and approved the final manuscript to be published.
Supported by Research Fund of the Education Department of Shaanxi Province, China, No. 2013JK0788.
Institutional Review Board StatementThe study was reviewed and approved by the First Affiliated Hospital of Xi ’an Medical College Institutional Review Board.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict of Interest StatementThe authors declare that there is no conflict of interest related to this study.
Data Sharing StatementNo additional data are available.
AcknowledgementsThe authors thank the participating team from Department of Gastroenterology and Physical Examination Department, First Affiliated Hospital of Xi ’an Medical College.