The Abernethy malformation is a rare congenital malformation defined by the presence of an extrahepatic portosystemic shunt. Although most patients are asymptomatic, clinical encephalopathy is present in 15% of cases. We present a patient with type 2 Aber-nethy malformation, hyperammonemia, and encephalopathy. Shunt closure was performed successfully using interventional angiography; however, hyperammonemia recurred 3 months later. The diagnosis of Abernethy malformation can be made easily, but the ideal patient management strategy has not yet been established. This is the first reported patient with recurrence of hyperam-monemia after interventional treatment; we discuss the therapeutic options for Abernethy malformation.
The Abernethy malformation is a rare congenital communication between the portal vein and systemic circulation; it was first reported by John Abernethy in 1793. According to the type of anastomosis and status of the portal vein, it is classified into 2 types.1 Type 1 is characterized by a complete absence of the portal vein and is predominantly (74% of cases) found in females.1-4 Type 1 malformation is further divided into subtypes A and B:5 in type 1A, the splenic vein and the superior mesenteric vein drain separately into the inferior vena cava; in type 1B, these veins form a common trunk. Partial shunts between the portal vein and the systemic venous circulation are defined as type 2; these allow for some potential portal per-fusion of the liver. Various clinical presentations and some congenital malformations are associated with these abnormal shunts:6 hepatic encephalopathy, portopulmonary syndrome, hepatic masses, cardiac defects, and vascular anomalies have all been reported.
We report a patient with type 2 Abernethy malformation and hepatic encephalopathy. Although she underwent successful interventional shunt closure, her hyperammon-emia recurred 3 months postoperatively. This is the first reported case of recurrent hyperammonemia after inter-ventional treatment; we discuss herein the therapeutic options for Abernethy malformation.
Case reportA 58-year-old female presented to the gastroenterology department of Shengjing Hospital (affiliated with China Medical University) with an 8-month history of dizziness, sleepiness. She was previously diagnosed, at another institution, with metabolic encephalopathy when she presented with elevated alanine transaminase levels without hepatic dysfunction. She was otherwise healthy, with a history significant only for a simple vocal-cord surgery. Physical examination revealed lower-extremity edema but no splenomegaly or evidence of ascites.
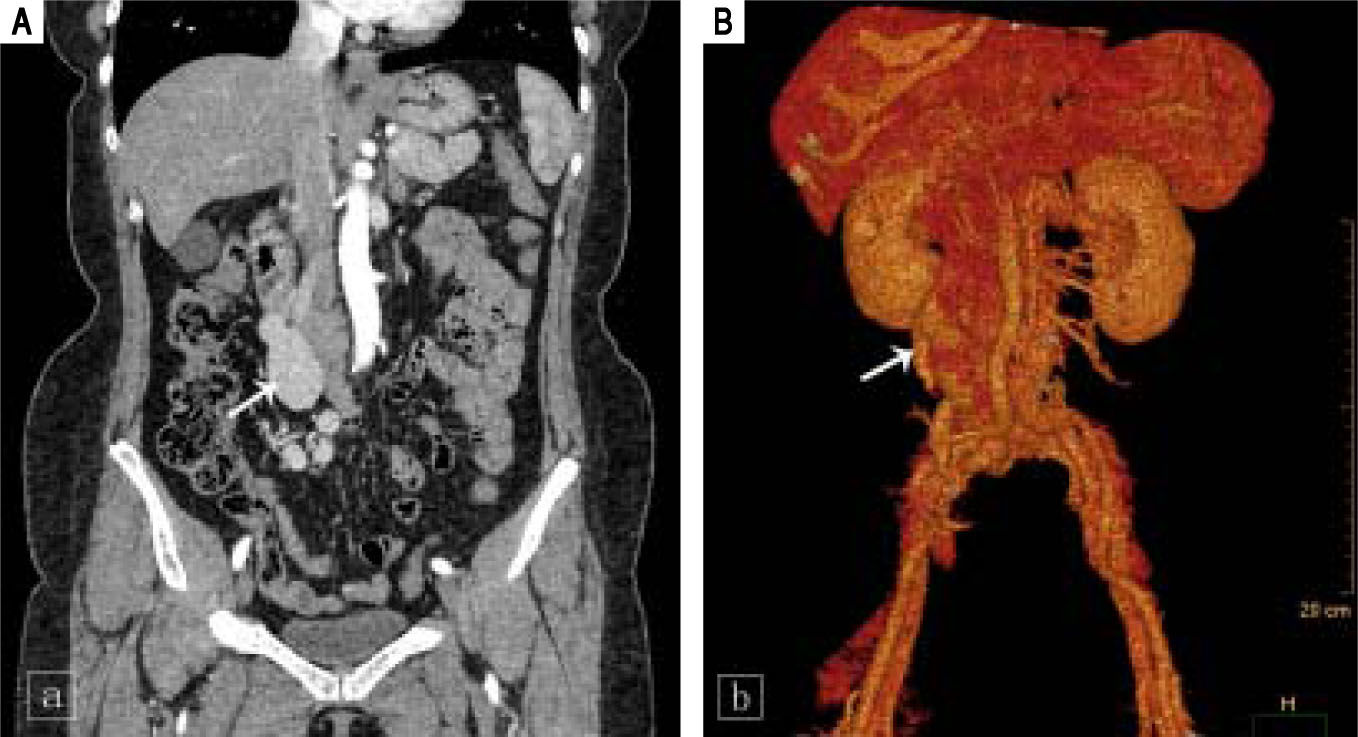
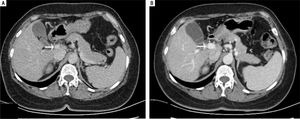
Laboratory testing revealed aspartate aminotransferase level of 39 IU/L (normal range, 5-34 IU/L), an alkaline phosphatase level of 180 IU/L (normal range, 40-150 IU/ L), a total bilirubin level of 24 μmol/L (normal range, 3.4-20.5 μmol/L), and an indirect bilirubin level of 15.7 μmol/ L (normal range, 3.4-11.9 μmol/L). Her white blood-cell count was 3.9 x 109/L (normal range, 3.5-9.7 x 109/L), her hemoglobin level was 116 g/L (normal range, 110-150 g/L), her platelet count was 180 x 109/L (normal range, 135-350 x 109/L), and her coagulation profile was normal. The tumor marker cancer antigen 19-9 (CA19-9) measured 58.47 U/ mL (normal range, 0-37U/mL). Serological evaluation for ceruloplasmin, hepatitis viruses A E, and immunological markers was negative, although her plasma ammonia level was 147.6 μmol/L (normal range, 9-33 μmol/L). Computed tomography of the abdomen was performed using a 64-row multidetector. Multiplanar reconstruction and 3-dimensional volume-rendered images confirmed the presence of an abnormal shunt between the inferior vena cava and the superior mesenteric vein; this shunt had a diameter of 33 mm (Figure 1).
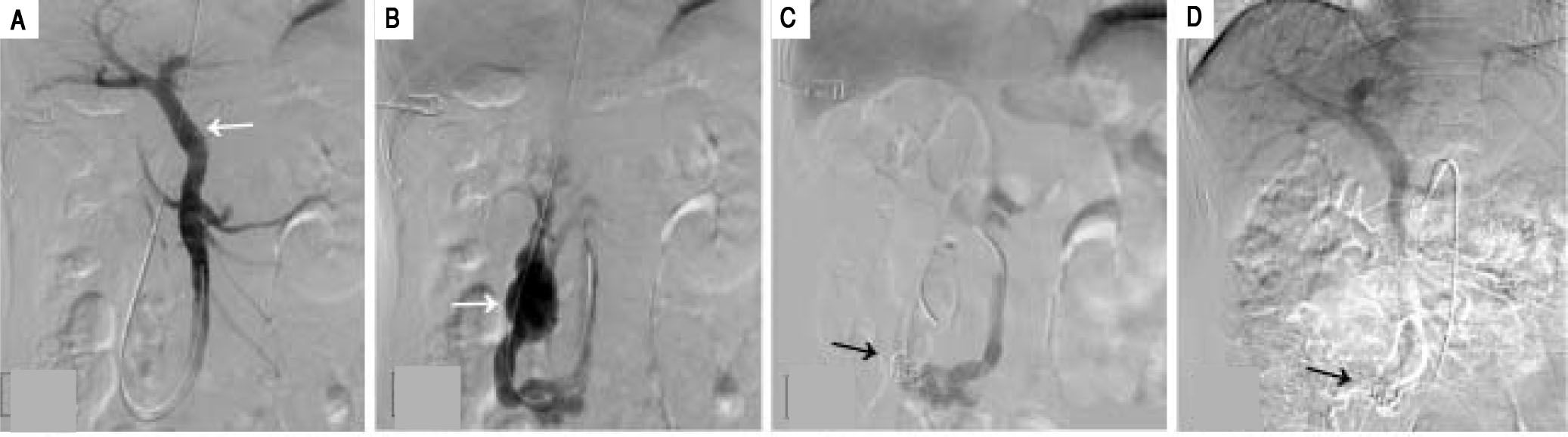
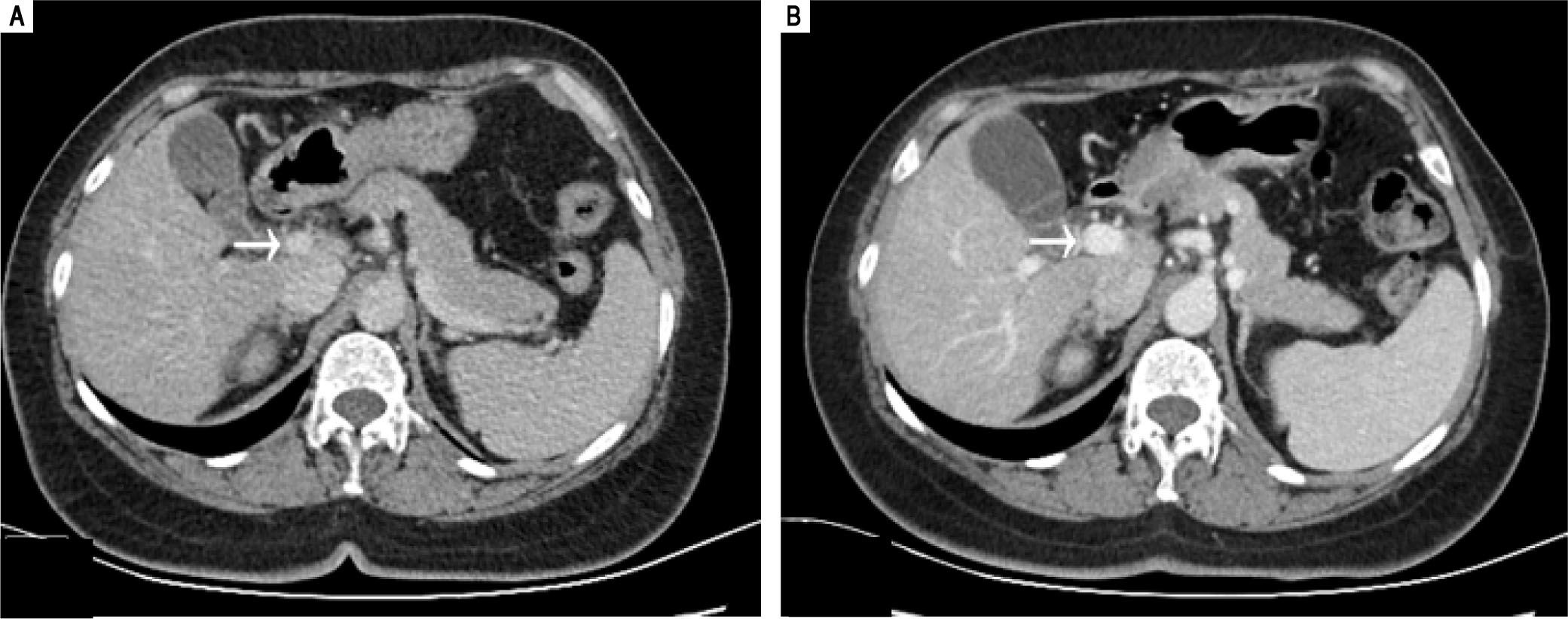
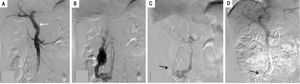
The patient subsequently underwent shunt closure, using interventional angiography (Figure 2). Her plasma ammonia level returned to normal range, with complete resolution of encephalopathy and the lower-extremity edema. However, about 3 months after surgery, routine follow-up examination revealed an elevated plasma ammonia level of 155 μmol/L; repeat angiography confirmed successful shunt closure and did not reveal any other portal-venous vessels that could explain the recurrence of hyperammonemia(Figure 2). However, contrast-enhanced CT of the liver revealed that the portal vein was 1.5 cm in diameter, larger than the presurgical value (Figure 3). Since she was asymptomatic, conservative observation was recommended. Over a year of follow-up, the serological evaluation for liver function was normal, while the plasma ammonia level was in the range of 60-80 μmol/L.
Angiographic images (A) shows the intrahepatic portal system (white arrow) before shunt closure; (B) shows the aberrant drainage branches of superior mesenteric vein into the inferior vena cava prior to closure (white arrow); (C) shows the successful closure of the shunt with coil embolization(black arrow); (D) repeat angiographic image shows the persistent closure of the shunt.
Abernethy malformation has a female dominance and is usually diagnosed during childhood.7 Hao, et al.,8 reviewed 101 previously reported patients with Abernethy malformation and found that 65.3% were female, 69.3% were younger than 18 years of age, and less than 10% had type-2 malformations.
Some patients remain asymptomatic throughout life.9 When symptoms do occur, the clinical manifestations are mainly associated with the hepatic shunt and associated congenital malformations. In the Abernethy malformation, diversion of the portal blood flow into the inferior vena cava leads to systemic hypertension, which can explain the leg edema seen in our patient. Metabolic and vasoactive substances bypass the liver through portosystemic collaterals and thus cause portopulmonary hypertension, hyper-ammonemia, and encephalopathy.10 Nodular liver lesions are seen in up to half of patients: these include benign focal nodular hyperplasia, hepatocellular adenoma, and regenerative nodules4,11 and may be explained by the absence of portal flow and compensatory increased hepatic arterial blood flow. Although most of these lesions are benign, there are several case reports in the literature describing the coexistence of hepatocellular carcinoma and hepatob-lastoma. Therefore, long-term follow-up and monitoring are recommended.12 Congenital hepatic shunts can also present with metabolic dysregulation, such as hypoglyc-emia, due to metabolic alterations in the liver.13
Despite the existence of a direct connection between the portal and systemic venous systems, hyperammonemia is found in only 26% of patients with the Abernethy mal-formation,14 and only 15% of all patients experience hepatic encephalopathy.15 A recent review identified 316 published cases and reported that hyperammonemia/neu-rological abnormalities occurred in 35% of patients with congenital portosystemic shunt.16 Hyperammonemia can be present without encephalopathy, especially in younger patients; clinical encephalopathy is more common at older ages. Possible explanations for this phenomenon are an age-dependent increase in sensitivity to deleterious metabolites and the impact of the extent of shunting, determined by the portal/systemic shunt ratio-a shunt ratio of more than 60% may predict the age of onset of encepha-lopathy.17,18
Current treatment options, including interventional or surgical shunt closure and liver transplantation, are described in case reports; however, the therapeutic experience with Abernethy malformation is still limited.19 The choice of strategy depends on the type of shunt, symptoms, complications, and comorbidities. Shunt occlusion is not feasible in patients with type 1, as the shunt is the only drainage route for the mesenteric venous blood. Thus, liver transplantation may be required to provide a patent portal system and to treat the metabolic abnormalities and liver disease in these patients.20 Kanamori, et al.21 previously reported on a patient with type 2 Abernethy malformation who required liver transplantation after developing postoperative portal hypertension and another portosystemic shunt. Thus, it is important to ensure adequate portal vein capacity and normal portal pressure prior to closure, in order to avoid postprocedure portal hypertension and subsequent mesenteric ischemia.22
The balloon shunt occlusion test shows a visible intra-hepatic portal system (IHPS) in patients with both type 1 and type 2 Abernethy malformation.23 This finding could represent small portal vein branches which cannot be seen on ultrasonography but can be visualized on shunt angiog-raphy.2,24 Kanazawa H, et al.,23 proposed a new classification based on IHPS hypoplasia (mild, moderate, and severe types) under shunt occlusion; this system guides the decision on whether to perform single-stage or 2-stage shunt closure. Single-stage closure is recommended for mild and moderate types with crescent-shaped portal veins and a portal venous pressure below 25 mmHg. This suggests that the intrahepatic portal vascular bed is able to accept sufficient blood flow immediately upon shunt closure. Patients with the severe type, with a small portal-triad area and a portal venous pressure over 25 mmHg, are candidates for either 2-stage shunt closure or liver transplantation. In the present case, the intrahepatic portal vein system was well displayed under angiography, similar to mild type of IHPS, which indicated the possibility of shunt closure. The balloon shunt occlusion test was not performed prior to operation and a direct shunt closure was carried out in this patient. The treatment was proved effective in both the improvement of symptoms and laboratory results. However, her plasma ammonia level was elevated again just 3 months later. Although repeat angiography showed persistent closure of the shunt, an enlarged portal vein was confirmed; thus, we conjectured that the closure of the original large shunt caused a sudden increased inflow into the portal vein, leading to portal hypertension, when then resulted in the development of new collaterals between the portal and venous vessels. As the diameter of shunt vessels grows with age, the difficulty of intervention increases. In our case, the large diameter of the longstanding aberrant vessel increased the difficulty of the original operation and the chance of complications. Therefore, it is necessary to carefully assess the severity of the hypoplasia of IHPS as well as the PVP under the occlusion test, and for cases with large abnormal vessel, simple shunt closure may not be the first choice.
In conclusion, the diagnosis of Abernethy malformation is challenging, as it is an uncommon congenital malformation with nonspecific clinical symptoms. However, patients with unexplainable hyperammonemia and hepatic encephalopathy require evaluation for a potential porto-systemic shunt. Since most significant presentations appear in older patients with large-diameter shunts, as well as in those with severe portal dysplasia, the incidence of postsurgical complications increases. Thus, it is necessary to evaluate the IHPS by angiography, using the shunt-occlusion test, and to measure the portal venous pressure under shunt occlusion in order to choose the optimal therapeutic approach. Moreover, in the present case, although the repeat angiography failed to show the new collaterals between the portal and venous veins, we couldn’t deny the existence of the recurrent shunts that might explain the recurrent hyperammonemia. Additionally, further tests should be done to explore the possible defects of urea cycle which might result in high ammonia. Postoperative clinical improvement, such as regression of benign liver masses and of pulmonary, cardiac, neurological, and renal complications, has been reported.24 However, there are patients described in the literature with hepatic lesions that did not improve by 12 to 24 months after shunt closure. These findings suggest that the malformation should be treated before a significant complication occurs and that long-term follow-up and monitoring for complications as well as malignancy are mandatory.23