Nowadays, the retreatment of patients with Hepatitis C virus (HCV) genotype 3 (GT3) especially cirrhotic, who have already been treated with regimens containing a NS5A inhibitor represents a challenge. Use a novel retreatment option for patients with a difficult approach. We present three case reports of retreatment with a new combination of Direct-acting antivirals (DAAs), Sofosbuvir, Elbasvir/Grazoprevir in patients with GT3 with a previous failure with Sofosbuvir/Ledipasvir. All the cases achieved sustained virologic response (SVR) at week +12 without adverse effects. In our experience, this combo may represent an effective and safe option for these patients.
Direct-acting antivirals (DAAs) are currently the treatment of choice for HCV infection1 with high SVR rates (over 90%). However, patients with HCV GT3 infection, especially the cirrhotic experienced patients have been associated with a low SVR. Nowadays, there are not too many treatment options for these patients, particularly for those who have already been treated with regimens containing a NS5A inhibitor.
ObjectivesStudy designWe present three case reports of retreatment with a novel combination of DAAs, Sofosbuvir (SOF) + Elbasvir (EBR) + Grazoprevir (GZP) in patients with GT3 HCV infection with a previous treatment failure with SOF + Ledipasvir (LDV).
These three patients were treated in the context of an HCV elimination program in the penitentiary ambit. Since fixed-dose combinations (FDC) may be the best way to improve drug adherence in difficult-to-treat populations, all patients were treated with LDV/SOF administered orally once daily without ribavirin and the therapy was administrated under direct observation.
ResultsCase 1A 48 years-old male, with a history of intravenous drug user (IDU) and HIV-coinfection (stage A2) treated with Emtricitabine/Rilpivirine/Tenofovir. He had an HCV-related chronic hepatitis GT3a naive, F2 fibrosis stage evaluated with transient elastography. He received treatment with LDV/SOF 12 weeks without Ribavirin. HCV-RNA was negative at end of treatment. He had a relapse at week +12 (4.16 log). We started treatment with the combination of SOF + EBV+GZP with Ribavirin (800mg) for 12 weeks. No adverse effects were observed, achieving SVR12.
Case 2A 50 years-old male, with a history of IDU and a HCV-related liver cirrhosis with portal hypertension (splenomegaly, without esophageal varices) and absence of previous liver-decompensation. Virological characteristics: GT3, naive. He received treatment with LDV/SOF 12 weeks without Ribavirin. Patient achieved undetectable HCV-RNA at the end of treatment. He relapsed at week +12 (6.76 log). We started treatment with the combination of SOF + EBV+GZP with Ribavirin (800mg) for 12 weeks. No adverse effects were observed achieving SVR12.
Case 3A 51 years-old male, with a history of IDU and HIV-coinfection (stage C2) treated with Emtricitabine/Tenofovir/Dolutegravir. He had a HCV-related liver cirrhosis, naive, GT3 with portal hypertension (esophageal varices grade I) without previous liver-decompensation. He received treatment with LDV/SOF 12 weeks without Ribavirin. He had HCV RNA undetectable at the end of treatment. He relapsed at week +12 (4.63 log). We started treatment with SOF+EBV+GZP with Ribavirin (800 mg) for12 weeks. At week2, the patient had asthenia and hemoglobin dropped two points, so that, the dose of Ribavirin was decreased to 600mg. The patient completed treatment with no more complications and achieved SVR12.
Resistance testing was performed on all 3 patient samples at baseline. Resistance-associated substitutions (RAS) in NS5A and NS5B were identified by population sequencing. The HCV NS5A and NS5B coding regions were amplified using standard RT-PCR and Ultradeep sequencing was done as previously described. We carried out a phylogenetic analysis of the nucleotide sequences. The HVR1 fragment (nucleotide positions 1156 to 1234) was chosen for sequence analysis because this domain exhibits a sufficiently high degree of variability. This allowed to distinguishing between HCV isolates of the same subtype. The HVR1 fragments isolated were aligned by using the SeaView program. Phylogenetic analysis showed that the 3 cases analyzed were recurrences and not reinfections. Finally, baseline RAS were detected in NS5A in 2/3 patients, presenting A30L and Y93H variants.
DiscussionTherapeutic options for patients with chronic hepatitis due to HCV GT3 who have failed previous DAA-containing regimens are limited. This is particularly important in those who have already been treated with NS5A inhibitors. These patients remain a special group with an unmet medical need. These patients are the most difficult to cure, particularly when they have some negative response cofactors such as cirrhosis.
There are few data with this combination of DAAs. The study C-SWIFT was a single-center trial in treatment-naïve patients with GT1-3 infection. All patients received EBR/GZP with SOF for 4-12 weeks. Among GT3-infected patients without cirrhosis, SVR12 was 100% after 12 weeks. SVR12 in GT3-infected patients with cirrhosis was 83% (10 of 12) after 12 weeks of treatment.2
This combo has been used in other genotypes, in the REVENGE study, patients with GT1-4 with failure to a prior therapy with SOF ± RBV + a NS5A inhibitor with documented presence of NS5A or NS3 RAS at failure. They were treated with SOF/EBR/GZP/RBV during 16 or 24 weeks effectively.3
Regarding the use of this combo in GT3 treatment-experienced patients, C-ISLE is a randomized study of SOF + GZP/EBR with or without RBV in cirrhotic patients. The study included 53 patients Peginterferon + Ribavirin experienced. Among GT3 treatment experienced SVR12 rate was 100% in the different groups of 12 or 16 weeks, with or without RBV. In this study, they observed a high efficacy regardless of the duration, the presence of RAS or patient condition. This combination is generally safe and well tolerated.4 Although the data in previously treated GT3 patients are scarce, they support this combination.
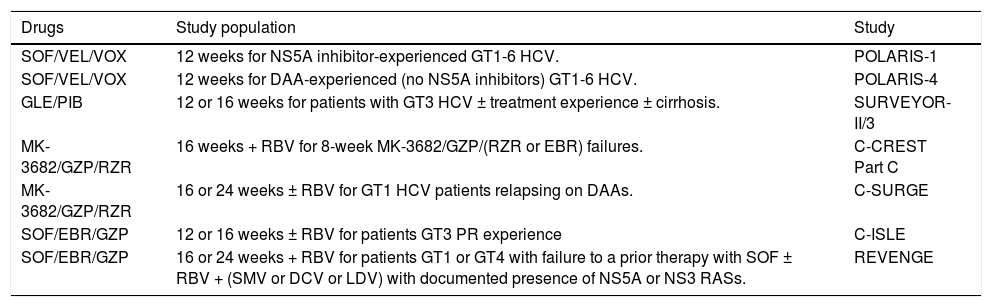
It is likely that in the near future, other combinations of AAD may play a role in these patients. The new combinations of drugs with promising results are summarized in the table 1
New retreat combinations.
| Drugs | Study population | Study |
|---|---|---|
| SOF/VEL/VOX | 12 weeks for NS5A inhibitor-experienced GT1-6 HCV. | POLARIS-1 |
| SOF/VEL/VOX | 12 weeks for DAA-experienced (no NS5A inhibitors) GT1-6 HCV. | POLARIS-4 |
| GLE/PIB | 12 or 16 weeks for patients with GT3 HCV ± treatment experience ± cirrhosis. | SURVEYOR-II/3 |
| MK-3682/GZP/RZR | 16 weeks + RBV for 8-week MK-3682/GZP/(RZR or EBR) failures. | C-CREST Part C |
| MK-3682/GZP/RZR | 16 or 24 weeks ± RBV for GT1 HCV patients relapsing on DAAs. | C-SURGE |
| SOF/EBR/GZP | 12 or 16 weeks ± RBV for patients GT3 PR experience | C-ISLE |
| SOF/EBR/GZP | 16 or 24 weeks + RBV for patients GT1 or GT4 with failure to a prior therapy with SOF ± RBV + (SMV or DCV or LDV) with documented presence of NS5A or NS3 RASs. | REVENGE |
POLARIS-1 (Table 1) had a cure rate by HCV GT3 of 91% (20/22) with the regimen of SOF-Velpatasvir (VEL)-Voxilaprevir (VOX).5
The efficacy and safety of glecaprevir (GLE)-pibrentasvir (PIB), was evaluated in the MAGELLAN-1, in noncirrhotic GT1 patients with prior DAA failure. A total of 50 patients were treated and only 2 experienced virological failure. In a second part of the study, 12 vs. 16 weeks of the dual FDC were tested, in patients with different genotypes, with SVR rates ranging from 80 to 100% depending on whether there is resistance to protease inhibitors (PI) and/or NS5a.6
With respect of the molecules of Merck, part of C-CREST trial (Table 1) enrolled 24 patients (2 GT1, 14 GT2, 8 GT3). All 23 patients who completed treatment achieved SVR, despite high rates of RAS.7
One aspect that is still under discussion is the need for a study of RAS before the rescue treatment of patients with a prior failure to AAD. This discussion is relevant, as more and more data suggest that the finding of RAS does not impact SVR as long as the regimen includes SOF. In this sense, relevant RAS to EBR, may limit its efficacy and lead to virological failure in some infected patients. However, in Spain, only 3.4% of patients had viruses with RAS that confer reduced susceptibility to EBR.8 Although this study suggests that in our country the combination of EBR/GZP could be used without analysis, we performed a RAS analysis in our patients. RAS was detected in two of our patients, although they had no impact on the virologic outcome, probably as a result of the combination with SOF.
The RAS analysis is recommended and it can be useful to guide the retreatment therapy.1
The last EASL HCV-Guidelines included rescue treatment with the combination of SOF, EBR/GZP for treatment failures NS5A inhibitor-containing regimen in genotype 1 and 4, but not in the genotype 3 as in our cases.1The retreatment strategies should include SOF due to its high genetic barrier. Importantly, we should associate drugs without cross-resistance with those previously used. As a difficult-to-treat population, we may add ribavirin whenever possible, which seems to increase the efficacy of antiviral treatment.1
Last but not least, an added advantage of this combination is the absence of significant interactions, particularly with antiretroviral agents, as happened in one of our patients. However, we must remember that GZP is a substrate of OATP1B1/3, so we should be aware of the co-administration of EBR/GZP with OATP1B1/3 inhibitors.
In summary, this case series represents a novel and effective retreatment option for patients with a difficult approach with extraordinary effectiveness results and few side effects.
Abbreviations- •
DAAs: direct-acting antivirals.
- •
EBR: elbasvir.
- •
FDC: fixed dose combinations.
- •
GLE: glecaprevir.
- •
GT: genotype.
- •
GT1: genotype 1.
- •
GT3: genotype 3.
- •
GT4: genotype 4.
- •
GZP: grazoprevir.
- •
HCV: hepatitis C virus.
- •
IDU: intravenous drug user.
- •
LDV: ledipasvir.
- •
MK 3682: uprifosbuvir.
- •
PI: protease inhibitor.
- •
PIB: pibrentasvir.
- •
PR: peginterferon ribavirin.
- •
RAS: resistance-associated substitutions.
- •
RBV: ribavirin.
- •
RZR: ruzasvir.
- •
SOF: sofosbuvir.
- •
SVR: sustained virologic response.
- •
VEL: velpatasvir.
- •
VOX: voxilaprevir.
The authors declares that there is no conflict of interest regarding the publication of this case report.
AcknowledgmentsThis research was partially supported by a grant from the Spanish Government (Integrated Projects of Excellence Call) PIE15/00079.