Hepatitis C virus infection (HCV) is a major cause of co-morbidity in people living with HIV (PLWHIV). The modes of HCV transmission in the local population of PLWHIV are still unclear. We conducted this study to identify risk factors for HCV transmission amongst PLWHIV in central Mexico.
Material and methodsWe enrolled HIV/HCV co-infected cases and HIV controls receiving care in two outpatient clinics in Mexico City. Structured questionnaires were applied, covering demographics, history of percutaneous exposures, sexual behaviors, self-reported STD and recreational drug use. The statistical analysis for between-group comparisons were multivariate logistic regression models to assess the risk factors associated with HCV co-infection. We limited the final analysis to men who have sex with men (MSM) to avoid confounders potentially related to HCV acquisition in other populations.
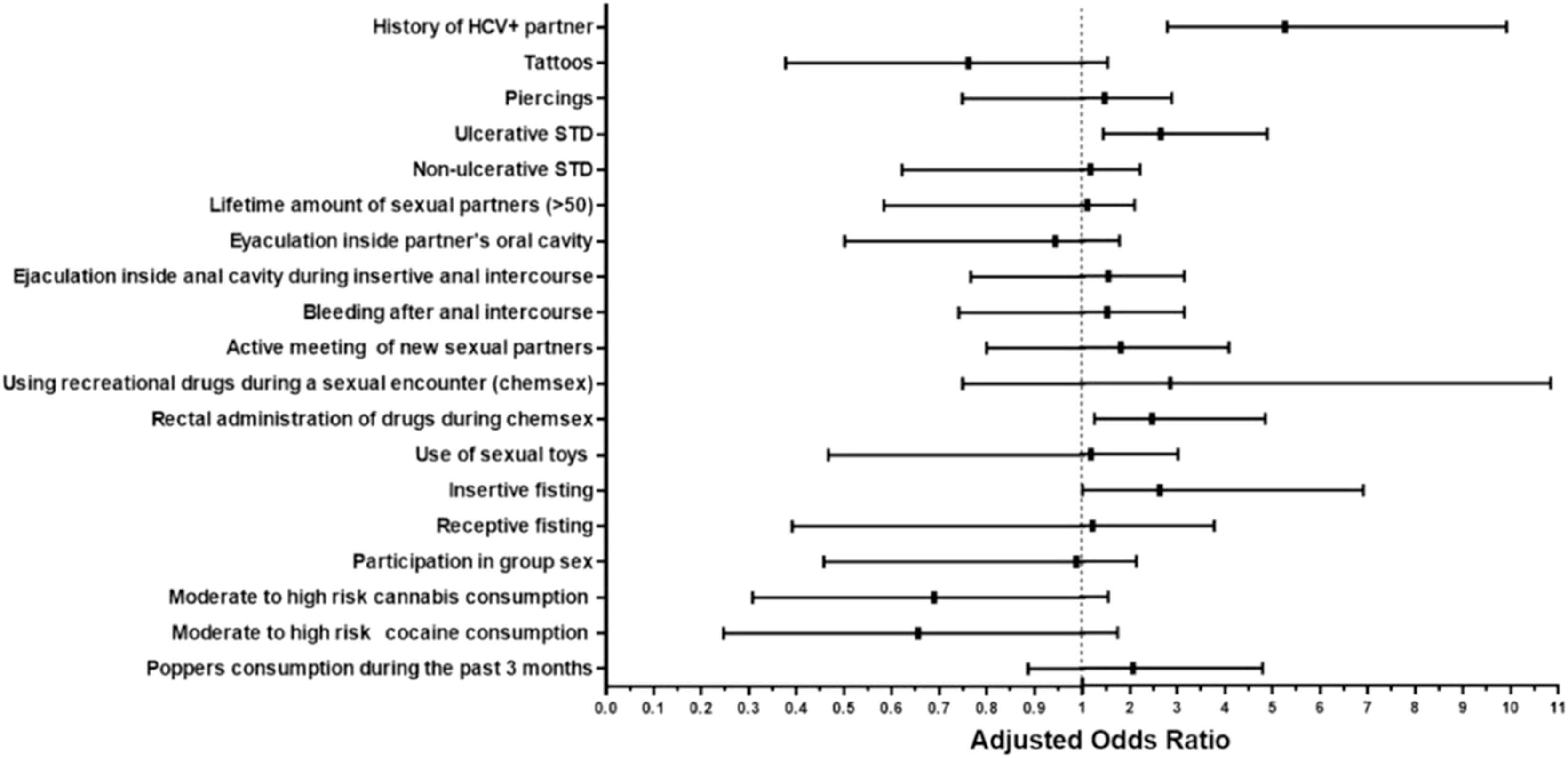
ResultsThree hundred and thirty-four MSM with HIV (175 with HCV co-infection and 159 without) were analysed. We did not identify percutaneous exposures as risk factors for HCV. Intravenous drug use (IVDU) occurred in two cases and one control case. Risk factors independently associated with acquiring HCV co-infection were: history of an ulcerative STD (aOR=2.65, 95%CI=1.44-4.88), a HCV positive partner (aOR=5.25, 95%CI=2.78-9.91), having practiced insertive fisting (aOR=2.62, 95%CI=1.01-6.90), and rectal administration of drugs during sex (aOR=2.46, 95%CI=1.25-4.84).
ConclusionsRisky sexual behaviors and chemsex seem to be the main drivers of HIV/HCV co-infection amongst PLWHIV in Central Mexico. IVDU and percutaneous exposures have a minor role in the local HCV epidemic. These findings highlight the importance of testing for HCV in sexually active MSMs.
Hepatitis C virus (HCV) infection remains one of the leading causes of liver disease globally [1]. HCV prevalence shows considerable variation across different populations, with multiple factors influencing local epidemics [2]. For example, HCV infection has a bimodal distribution globally, affecting older individuals (>50 years) and younger (20-40 years). Older individuals are mostly infected due to a recent or past history of bloodborne exposures (mainly related to iatrogenic procedures), while infection in younger individuals is mainly driven by intravenous drug use (IVDU) [ 3, 4]. Unprotected sex (particularly when coupled with chemsex) is a known route of HCV transmission amongst people living with human immunodeficiency virus (PLWHIV) and men who have sex with men (MSM) [ 5, 6].
HCV/HIV co-infection increases the rate of progression to advanced liver fibrosis as well as the risk of developing hepatocellular carcinoma [3]. The global prevalence of HIV/HCV co-infection is estimated in more than 2.3 million people [6, 7]. The highest burden of co-infection is located in Eastern Europe and Central Asia, mainly due to the high rate of transmission occurring in people who inject drugs (PWID) [6–8]. The quality of the epidemiological data varies across countries, with most developing nations lacking robust data. In Latin America, HIV/HCV co-infection remains an understudied subject. Little information exists regarding the incidence and risk factors associated with HCV transmission and the existing information mainly focuses on populations with a high prevalence of IVDU [9].
The incidence of HCV in Mexico increased between 2008 and 2019. During this period, HCV incidence increased predominantly in the younger male population, with a male-to-female ratio of 1.73 to 1 [10]. In 2019, the majority of the new cases reported (76.3%) occurred in two geographic areas of the country: the north-western states (Baja California) and Mexico City and its surrounding areas (Central Mexico) [10]. Epidemiological data indicates that substantially different phenomena drive both epidemics. Cities on the northern Mexican border are known hotspots for drug trafficking, [11]. with high reported rates of IVDU (1.7%-4.9%) [12– 14]. In contrast, in the country's central region, IVDU is uncommon (<0.1%) [15]. and thus an unlikely cause for the increasing incidence of HCV infection. This, in turn, suggests that other conditions may be associated with HCV transmission in the growing epidemic occurring in younger people in Central Mexico [10].
In developed countries, high-risk sexual behavior has been described as a risk factor for HCV transmission in younger people, predominantly in MSM and PLWHIV with no history of IVDU. In this particular population, risk factors associated with HCV infection were the use of recreational drugs during sexual intercourse (chemsex) and sexual practices related to anal trauma (for example, during fisting) [16, 17]. In some regions of Mexico and Latin America, high-risk sexual behaviors could potentially co-exist with parenteral exposures and unsafe medical practices [18]. Identifying behaviors and exposures associated with the transmission is essential to promote early testing and treatment in the population at risk of infection. In this case-control study, we decided to apply a structured questionnaire to identify risk factors associated with HCV transmission in PLWHIV, focusing on demographic characteristics, history of percutaneous exposure to blood, sexual behaviors, and drug use.
2Materials and methods2.1Study settingsWe conducted the study in two large HIV outpatient clinics in Mexico City: the Clinica Especializada Condesa (CEC) and the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ). The CEC is a primary-care center for PLWHIV and the largest provider of antiretroviral treatment (ART) in Mexico. It offers screening for sexually transmitted diseases (STD) screening services and general HIV care in Mexico City and currently follows up over 14,000 adults across two different campuses. The INCMNSZ is a tertiary-care center; it provides hospitalization services and outpatient care for adults and currently follows up approximately 2,100 adults with HIV infection.
2.2Study designWe conducted a prospective case-control study between January 2016 and October 2018. Participants were recruited from the patient population of both centers and were selected by convenience sampling, stratifying by study site. We used a case-cohort strategy for the selection of controls. We confirmed the negative HCV status of controls with either the presence of a non-reactive anti-HCV test in the past six months or a negative HCV recombinant immunoblot assay in case the patients had a positive HCV ELISA and a negative HCV RNA viral load. Exclusion criteria for both groups included refusing to sign an informed consent or being younger than 18 years old at the moment of Hepatitis C diagnosis. We originally planned for our study to include all men and women with HCV/HIV co-infection, but after we identified that a significant majority of participants were MSM (>90%), we decided to limit the study groups to the MSM population and transgender women to avoid confounders potentially related to HCV acquisition in other populations. Thus, persons who did not identify themselves as MSM or transgender women were excluded from the analysis. Also, due to the low numbers of transgender women in the study and the similarities that this population shares on HCV and HIV risk factors with MSM, we decided to analyze the data from this population in conjunction with the main database of MSM. All patients were linked to HCV care services after their recruitment.
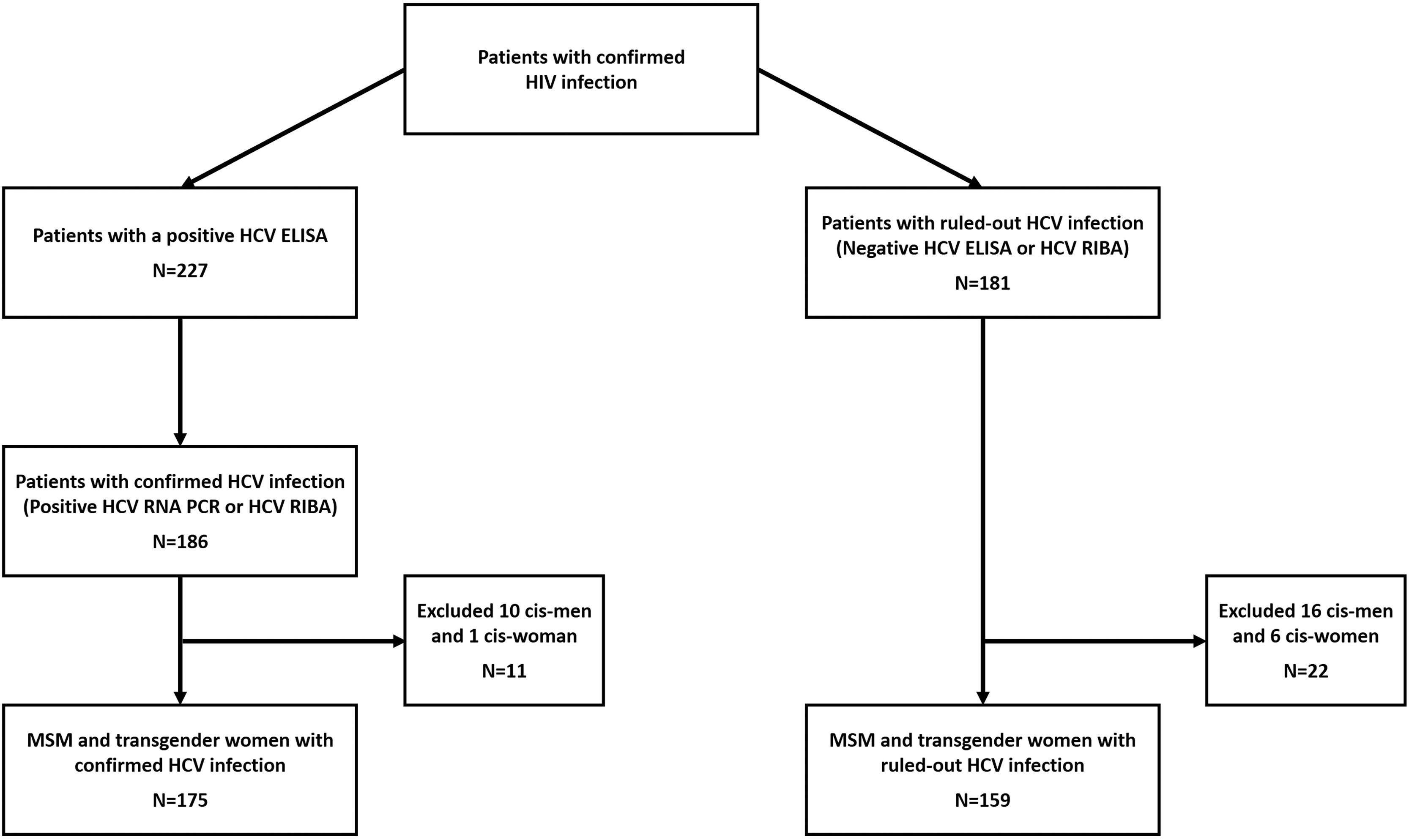
2.3Laboratory proceduresAccording to local guidelines, HIV-infected patients are routinely screened for anti-HCV antibodies (Architect i2000 system, Abbott Diagnostics, Wiesbaden, Germany) at their first visit after HIV diagnosis, whenever clinical suspicion suggests a possible infection, and ideally as part of routine care every year after that [19]. We invited patients with a positive anti-HCV assay to participate in the study. We confirmed HCV infection by quantitative RNA test (COBAS TaqMan HCV Test, v2.0, Roche Diagnostics, Pleasanton, CA). HCV genotyping was performed in all patients with an HCV viral load >5,000 IU/ml. HCV genotype determination methods were processed by a hybridization-based line probe assay (VERSANT LiPA; Healthcare SIEMENS, Munich, Germany). Stored serum samples were tested by a recombinant immunoblot assay (RIBA) [INNO-LIA HCV Ab III, Zwijndrecht, Belgium] if the patient had a reactive anti-HCV test and undetectable HCV RNA. We considered RIBA-positive cases as true-positive HCV co-infection and RIBA negative cases as false positives (we further reclassified and included these patients in the HIV mono-infection control group) (Fig. 1).
Group classification algorithm.
Here we show the classification algorithm for the study. Patients in the case group were included if they had a positive HCV ELISA and either a positive HCV RNA viral load or a positive HCV RIBA in case the viral load was negative. We confirmed the negative HCV status of controls with either a non-reactive anti-HCV ELISA or a negative HCV RIBA (in patients with a positive HCV ELISA and negative HCV RNA negative, this way, we classified the original HCV ELISA as false positive). HCV: Hepatitis C virus. ELISA: Enzyme-linked immunosorbent assay. RNA PCR: Ribonucleic acid polymerase chain reaction. RIBA: Recombinant immunoblot assay.
A standardized questionnaire was applied to all subjects after written informed consent was given. The questionnaire covered sociodemographic information, previous percutaneous exposures, sexual behavior and history of STDs. Additionally, we evaluated the history of substance abuse using the World Health Organization's Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) Version (V) 3.0 questionnaire [20].
2.5Sample size calculationAccording to the literature on the prevalence of risky sexual behaviors (specifically fisting, history of group sex participation, sex in public venues, recent STD and use of recreative drugs during sexual activities) among HIV-positive men in similar study settings, we assumed a prevalence of suspected exposures of among controls ≥10% [15]. To detect an Odds Ratio of ≥ 2.5 with a power of 80% and a type-I error of 5% (two-sided), we calculated a sample size of 292 participants (146 per group).
2.6Statistical analysisWe used mean and standard deviation, or median and interquartile range (IQR), as appropriate to summarize continuous variables. We used frequencies and simple proportions to summarize categorical variables. We performed a bivariate analysis to evaluate the associated odds ratio of the different variables with HIV/HCV co-infection. Afterward, we carried out a multivariate logistic regression to evaluate the adjusted odds ratio (aOR) of all demographic variables and all variables that returned a p-value ≤0.1 in the bivariate analysis, removing variables with a variable inflation factor >5. Due to the fact that most variables relating to sexual behaviors and substance use are intimately correlated (making co-linearity and over adjustment a bigger issue than normal), we made the decision to lower the normal thresholds of P-value <0.2 and of VIF <10 in order to reduce the possibility of collinearity in the multivariate analysis [21, 22]. For the multivariate analysis, we handled missing data using multiple chained equations to impute missing data with 10 imputations. All analyzes were performed using STATA v14.0 (StataCorp, Texas).
2.7Ethical statementThis study was approved by the Institutional Review Board of the National Institute of Medical Science and Nutrition Salvador Zubiran (registry: 1870) in Mexico City and was conducted according to the principles of the Declaration of Helsinki. All participants gave written informed consent before blood sample donation.
3Results3.1Study participantsWe identified 186 patients with a confirmed diagnosis of HIV/HCV (cases) and 181 patients with a confirmed HIV mono-infection (controls). We formed the case group with 128 patients (68.82%) from CEC and 58 (31.18%) from INCMNSZ, and the control group with 101 (55.80%) from CEC and 80 (44.20%) from INCMNSZ. We excluded 11 patients from the case group (10 cisgender men and 1 cisgender woman) and 22 patients from the control group (16 cisgender men and 6 cisgender women). The number of excluded patients was similar in both groups (10.71% vs. 14.97%, p=0.212). We describe the patient inclusion process in Fig. 1.
3.2Demographic dataThe demographic characteristics of included participants are summarized in Table 1. There were no significant differences amongst groups when comparing demographic data between both recruitment sites. The totality of participants identifies themselves as Hispanic/Latino.
Patients demographic characteristics
All values are n(%), unless otherwise noted.
Of the 175 patients with active HCV infection, 154 (88%) had their HCV virus genotype characterized. We did not perform an HCV genotype in 21 patients (12%) due to low HCV viral loads (<5000 copies/ml). The most common genotype was 1a (65.61%), followed by genotype 4 (13.64%) and genotype 1b (8.44%).
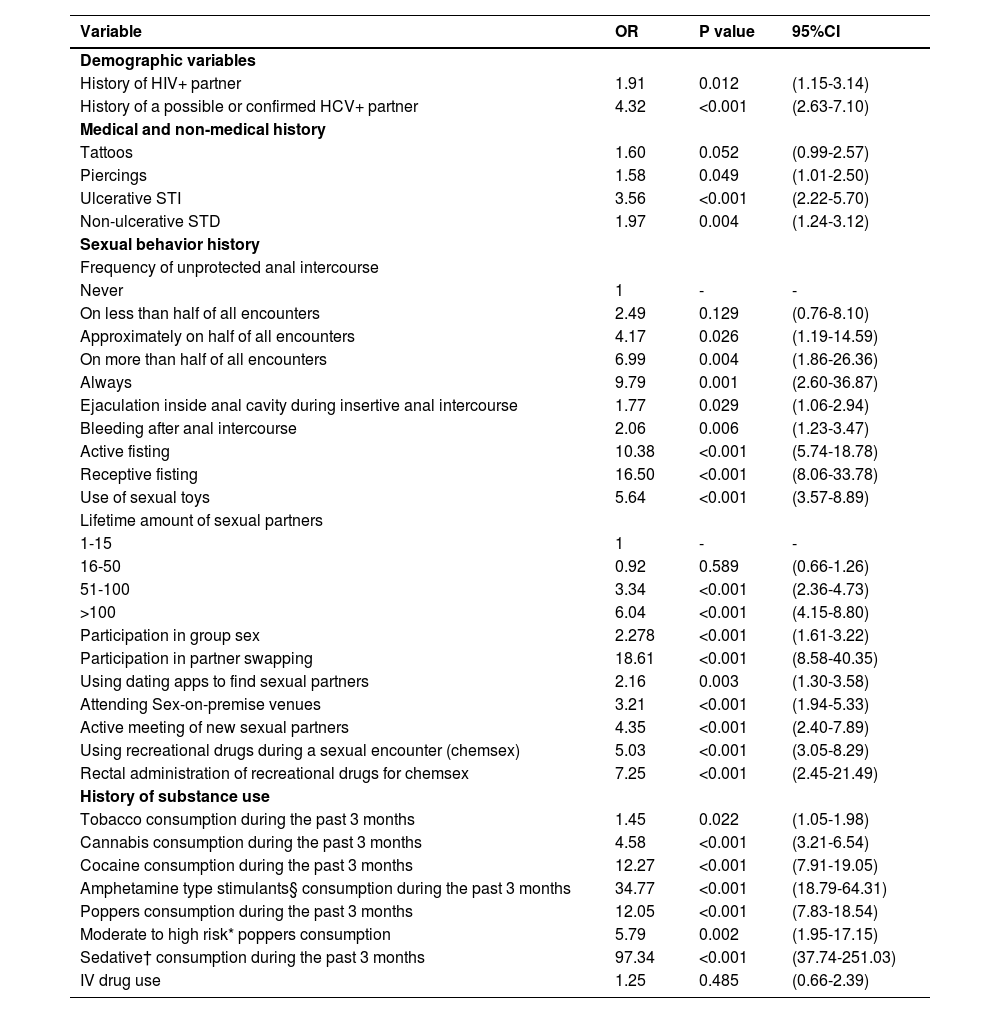
3.4Bivariate analysis: risk factors for HIV/HCV co-infectionWe show the results of the bivariate analysis in Table 2. We found no correlations with HIV/HCV co-infection when analyzing percutaneous exposures (aside from a history of body piercing). As part of the questionnaire, we asked the patients if they remembered if the tattoo or piercing was performed using sterile equipment and with new, disposable needles. There were no reports of any tattoo or piercing occurring under unsafe circumstances. IVDU was not correlated with HIV/HCV co-infection, and was generally uncommon amongst cases (n=2, 1.14%) and controls (n=1, 0.63%). A self-reported history of ulcerative STD (mainly syphilis, reported by 92.66% of patients in the case group with an STD and 96.43% of controls with an STD) was positively correlated to having HIV/HCV co-infection.
Bivariate analysis for factors associated with HIV/HCV co-infection: significantly associated variables
| Variable | OR | P value | 95%CI |
|---|---|---|---|
| Demographic variables | |||
| History of HIV+ partner | 1.91 | 0.012 | (1.15-3.14) |
| History of a possible or confirmed HCV+ partner | 4.32 | <0.001 | (2.63-7.10) |
| Medical and non-medical history | |||
| Tattoos | 1.60 | 0.052 | (0.99-2.57) |
| Piercings | 1.58 | 0.049 | (1.01-2.50) |
| Ulcerative STI | 3.56 | <0.001 | (2.22-5.70) |
| Non-ulcerative STD | 1.97 | 0.004 | (1.24-3.12) |
| Sexual behavior history | |||
| Frequency of unprotected anal intercourse | |||
| Never | 1 | - | - |
| On less than half of all encounters | 2.49 | 0.129 | (0.76-8.10) |
| Approximately on half of all encounters | 4.17 | 0.026 | (1.19-14.59) |
| On more than half of all encounters | 6.99 | 0.004 | (1.86-26.36) |
| Always | 9.79 | 0.001 | (2.60-36.87) |
| Ejaculation inside anal cavity during insertive anal intercourse | 1.77 | 0.029 | (1.06-2.94) |
| Bleeding after anal intercourse | 2.06 | 0.006 | (1.23-3.47) |
| Active fisting | 10.38 | <0.001 | (5.74-18.78) |
| Receptive fisting | 16.50 | <0.001 | (8.06-33.78) |
| Use of sexual toys | 5.64 | <0.001 | (3.57-8.89) |
| Lifetime amount of sexual partners | |||
| 1-15 | 1 | - | - |
| 16-50 | 0.92 | 0.589 | (0.66-1.26) |
| 51-100 | 3.34 | <0.001 | (2.36-4.73) |
| >100 | 6.04 | <0.001 | (4.15-8.80) |
| Participation in group sex | 2.278 | <0.001 | (1.61-3.22) |
| Participation in partner swapping | 18.61 | <0.001 | (8.58-40.35) |
| Using dating apps to find sexual partners | 2.16 | 0.003 | (1.30-3.58) |
| Attending Sex-on-premise venues | 3.21 | <0.001 | (1.94-5.33) |
| Active meeting of new sexual partners | 4.35 | <0.001 | (2.40-7.89) |
| Using recreational drugs during a sexual encounter (chemsex) | 5.03 | <0.001 | (3.05-8.29) |
| Rectal administration of recreational drugs for chemsex | 7.25 | <0.001 | (2.45-21.49) |
| History of substance use | |||
| Tobacco consumption during the past 3 months | 1.45 | 0.022 | (1.05-1.98) |
| Cannabis consumption during the past 3 months | 4.58 | <0.001 | (3.21-6.54) |
| Cocaine consumption during the past 3 months | 12.27 | <0.001 | (7.91-19.05) |
| Amphetamine type stimulants§ consumption during the past 3 months | 34.77 | <0.001 | (18.79-64.31) |
| Poppers consumption during the past 3 months | 12.05 | <0.001 | (7.83-18.54) |
| Moderate to high risk* poppers consumption | 5.79 | 0.002 | (1.95-17.15) |
| Sedative† consumption during the past 3 months | 97.34 | <0.001 | (37.74-251.03) |
| IV drug use | 1.25 | 0.485 | (0.66-2.39) |
In terms of sexual risk behaviors, all of the following were not correlated with HIV/HCV co-infection: age of sexual initiation, predominant sexual role, or behaviors related to oral sex. The vast majority of sexual behaviors related to unprotected anal intercourse and anorectal trauma were associated with HIV/HCV co-infection, with the following variables having significant correlations in the bivariate regression analysis: Bleeding after anal intercourse, history of sexual partner ejaculating in the anal cavity, use of sex toys before or during sexual intercourse, attending sex-on-premise venues, use of mobile electronic applications to meet sexual partners, group sex and, insertive and receptive fisting. None of the participants that reported practicing fisting (either insertive or receptive) referred to using gloves during the act. We found significant correlations between the consumption of all interrogated substances and HIV/HCV co-infection. Accordingly, cases scored higher in the ASSIST evaluation. Also, we found that the use of recreational drugs during sexual intercourse and the anal administration of drugs were both strongly correlated with HIV/HCV co-infection. A complete list of all the analyzed variables can be found in supplementary table 1.
3.5Multivariate analysis: risk factors for HIV/HCV co-infectionThe resulting adjusted odds ratios (aOR) of the multivariate logistic regression are shown in Fig. 2. The following variables were independently associated with HIV/HCV co-infection: self-reported history of an ulcerative STD (aOR=2.65, 95%CI=1.44-4.89), having practiced insertive fisting (aOR=2.63, 95%CI=1.01-6.91), and the rectal administration of drugs during sex (aOR=2.47, 95%CI=1.26-4.85). History of a possible or confirmed HCV+ partner (aOR=5.26, 95%CI=2.79-9.91) was also an associated factor. The following variables had a variable inflation factor (VIF) >5 and thus were removed from the multivariate analysis: age (VIF=19.79) and alcohol consumption during the past three months (VIF=52.90).
4DiscussionWe conducted a case-control study that evaluated factors associated with HIV/HCV co-infection in PLWHIV in Mexico City in MSM and the transgender population. We found that the history of an ulcerative STD, specific sexual practices related to anorectal trauma (insertive fisting and anal administration of recreational drugs), and the history of having an HCV-infected partner, were all independently associated with HIV/HCV co-infection. All these are well-described risk factors for the sexual transmission of HCV amongst MSM [4, 6, 17]. We also found an almost negligible prevalence of IVDU in our study, which is consistent with studies on the prevalence of drug use in Central Mexico, in which IVDU is infrequent [15]. Both of these findings suggest that in this population, high-risk sex behaviors represent the major route for HIV/HCV co-infection, which is in line with results reported in other regions by other authors [4, 16, 17]. Additionally, not reported in other studies, our study found that the use of rectally administered recreational drugs (mainly cocaine) for chemsex was an important factor associated with HIV/HCV co-infection.
Even though a great amount of unprotected sexual practices related to anorectal trauma that are traditionally identified as risk factors for HCV acquisition in this population (like receptive fisting) [17]. were not found to be independently associated with HCV transmission in our study, that does not mean that we think that they are not important factors in increasing the risk for HCV infection in MSM or transgender women. Due to the nature of our multivariate analysis and the high amounts of interactions between the different variables, we think that the variables that were found to be independent risk factors either simply represent the riskiest behaviors or just reliably identifies patients that perform multiple high-risk sexual behaviors.
The prevalence of non-injecting recreational drug use (cocaine, cannabis, amphetamines, and inhaled nitrites) in the HIV/HCV co-infected population (especially in the context of chemsex) was high. In contrast to reports from developed countries, where drugs such as methamphetamines and Gamma-hydroxybutyrate (GHB) are the most frequently used substances for chemsex [4, 17], in our context, cannabis, cocaine, and inhaled nitrites were the most frequently reported [15]. We consider this relates to the more widespread access and lower prices of those substances. Also, we found a significant association in the bivariate analysis with the use of inhaled nitrites and HIV/HCV co-infection. Inhaled nitrites have been associated with STD, such as Neisseria gonorrhoeae, Chlamydia trachomatis, and HIV.[23–25]. Inhaled nitrites are potent vasodilatory substances. Additionally, nitrites may decrease pain perception and favor microtears in the rectum, which may be less recognized during anal intercourse [26, 27]. The lack of IVDU in our study contrasts sharply with studies from cities on the northern Mexican border, which report a high prevalence of IVDU among the co-infected population, which is a common pattern described in other regions with high IVDU prevalence [28, 29] These data suggest differences in the main drivers of HCV transmission among HIV-infected populations: one pattern being dominated by IVDU and the other by sexual behaviors. Both patterns seem to be represented geographically in Mexico, with the IVDU-dominant pattern located in the northern regions and sexual transmission in the central region [28–31].
The majority of HCV infections in the assessed population were due to genotype 1 subtype 1a, and genotype 4 emerged as the second most prevalent subtype in typable samples. The genotype distribution in our study showed differences from previous studies in the general population of the country in which there is a minimum proportion of genotype 4 infections where the prevalence ranged from 0.3-0.5% [32, 33]. Local transmissions occurring in sexual networks of MSM with high-risk sexual behaviors may explain the transmission of this genotype, which has been described previously in the Netherlands, where the introduction of genotype 4 infections was related to clustered transmission in HIV/HCV co-infected MSM [34].
Our study has several limitations. We consider the questionnaire and its application to be the most important biases in our study for several reasons. First, when the questionnaires were applied, participants were already chronically infected with HCV, which may have a significant recall bias. Second, the interviewers were not blinded to the HCV serostatus of the participants, and thus observer bias cannot be ruled out. Finally, the information shared by the study participants in their interviews contained sensitive and personal information, potentially stigmatizing, which in turn could have derived from social desirability bias. The social desirability bias was partially attenuated by directly involving experienced personnel from the department of mental health of CEC (A. Harumi Hirata-Hernández) in both the design of the questionnaire and the training of the interviewers.
Another limitation of the study is the important selection bias of the study population. The study was carried out in the mostly uninsured population, which receives care from the National Commission of Social Health Protection (Seguro Popular) [35]. This limits the possible socioeconomic and epidemiological profiles of the interviewed subjects. Even though both populations have very similar epidemiological profiles, the fact that a more significant proportion of controls was recruited from the INCMNSZ than from the CEC could, in turn, introduce further bias. Also, we used clinic-based controls, which adds another layer of bias, as we are likely using patients with more co-morbidities and with a better linkage-to-care ratio than the general population.
The fact that we limited our study to MSM and transgender women also limits our ability to draw wider conclusions relating to HCV acquisition in cisgender patients. The associated risk factors for HCV transmission for cis-gendered men and women were different than those found in MSM and thus would have needed separate multivariate models in order to avoid interfering with the confidence intervals of the associated risk factors for HCV transmission in MSM and transgender women. The sample size of cis-gendered men and women that we obtained in the study was <5% of the total sample. This makes it extremely unlikely that we could have had a sufficiently large sample size to find significant risk factors.
We do not know the exact time of HCV acquisition in most patients, so drawing conclusions on which CD4 count or antiretroviral therapy was associated with the event of HCV acquisition would be subject to major bias. Finally, the results of this study may not be generalizable to other Latin-American settings with higher rates of IVDU.
5ConclusionsOur findings strongly support previous knowledge that sexual transmission is the driver route for HIV/HCV co-infection in populations with low IVDU prevalence. We found that HCV/HIV co-infected men and transgender women are more commonly engaged in chemsex and other high-risk sexual behaviors that increase the risk of inadvertent blood exposure. This expands the knowledge of the ongoing HCV epidemic amongst men and transgender women in central Mexico, in which great preventive efforts targeting risky behaviors and effective linkage to care are urgently needed.
Author contributionsLERG, APM, JFSA and JGSM conceived and designed the study, ACZ, LERG performed the statistical analysis. ACZ, KAMZ, APC, IZT, ESA assisted with database construction and management, AHHH Questionnaire design, Recruitment of participants: LERG, KAMZ, APC, ACZ, IZT, ESA, Clinical supervision: JGSM, Wrote the paper: LERG, ACZ, APM, JGSM.
FundingThe case-control study was funded with internal resources of the Department of Infectious Diseases (INF/1870), National Institute of Medical Science and Nutrition Salvador Zubirán of Mexico City. The determination of HCV viral load and genotype was supported by the Centre for Research in Infectious Diseases, National Institute of Respiratory Diseases of Mexico City. We thank the American Association for the Study of Liver Diseases (AASLD) for The Maribel Rodriguez-Torres Memorial Travel Award provided.
Data availability statementThe study questionnaire may be available upon request by emailing the corresponding author.
We gratefully acknowledge all patients, caregivers, and laboratory personnel involved in the Department of Infectious Diseases of the National Institute of Medical Science and Nutrition Salvador Zubirán and both campuses of Specialized Clinic Condesa that make our work possible. We further acknowledge the following physicians: Jeremy Bernardo Cruz- Islas (Condesa Specialized Clinic, México City) for help in designing the questionnaire; Juan José Calva-Mercado (National Institute of Medical Science and Nutrition Salvador Zubiran, Mexico City) for his valuable assistance during the study design.