Background. Serum levels of cystatin C, an endogenous inhibitor of cysteine proteases, provide an alternative method to creatinine-based criteria for measuring glomerular filtration rate. Preliminary data suggested that serum cystatin C levels parallel with the stage of liver fibrosis in chronic liver disorders. Our aim has been to evaluate the possible role of serum cystatin C as a marker of liver fibrosis in hepatitis C virus (HCV)-induced chronic liver disease. Material and methods. 100 consecutive patients (56 men, mean age 51.2 ± 9.5 yrs) with HCV-induced chronic liver disease, scheduled for their first liver biopsy and naive for antiviral therapy were included. Liver fibrosis was evaluated with the METAVIR score. Serum cystatin C and standard laboratory tests were measured simultaneously. Patients with ethanol abuse (> 50 g/day), HBV or HIV coinfection or plasma creatinine ≥ 1.20 mg/dL were excluded. In addition, a second group of 16 patients fulfilling the same requisites and diagnosed with HCV-induced compensated cirrhosis by clinical evidence of portal hypertension was included. Results. Serum cystatin C levels significantly increase from F0 to F2 fibrosis stages, remained stable in F3 and F4 stages and increased again in the group of non-biopsied compensated cirrhosis. Serum cystatin C levels were higher in patients with moderate-advanced necroinflammation in the liver biopsy. Conclusion. Serum cystatin C level may reflect current fibrogenic and necroinflammatory activities in chronic HCV-induced liver disease with normal renal function but can not be considered as a non-invasive marker of liver fibrosis.
Progressive fibrosis is the main prognostic factor of an unfavourable course in hepatitis C virus (HCV)-induced chronic liver disease. Liver biopsy still remains as the best standard for evaluating the stage of fibrosis in chronic hepatitis C (CHC), but it is far from being the gold standard we need due to sampling errors and inter-and intraobserver variability.1 Non-invasive markers of ongoing active fibrogenesis (class I biomarkers) and indirect markers reflecting the liver amount of collagen (class II biomarkers) have been proposed as tools to evaluate the stage of fibrosis in CHC. In addition, modified imaging tests, as transient elastography, have been proposed to evaluate the liver stiffness as an indirect marker of liver fibrosis, but none has still shown enough sensitivity and specificity to be used instead of liver biopsy, mainly to differentiate between intermediate stages of fibrosis.2,3
Cystatin C is a low molecular weight nonglycosylated protein that acts as a cysteine protease inhibitor.4 It is produced at a constant rate in all nucleated cells, eliminated by glomerular filtration and reabsorbed and catalysed in proximal tubular cells.5 Serum levels of cystatin C are independent of age, sex and muscle mass and are not influenced by bilirubinemia, inflammation or neoplasia.6,7 Serum cystatin C could provide an alternative method to creatinine-based criteria of measuring glomerular filtration rate, indeed in patients with chronic liver disease, with independence of the fibrosis stage. However, two preliminary studies suggested that serum cystatin C levels paralleled the stage of liver fibrosis in several chronic liver diseases.8,9 This finding represents a potential bias for the assessment of glomerular filtration rate in cirrhotic patients, but offers a new possibility to find an indirect marker of liver fibrosis.
The aim of this study has been to analyse the possible relationship between the stage of fibrosis and serum cystatin C levels in a group of patients with HCV-induced chronic liver disease with normal renal function (as measured with serum creatinine levels), excluding those with decompensated cirrhosis, hepatocellular carcinoma or other causes of liver disease different from HCV infection.
Material and MethodsBetween December 2007 and December 2011, 100 untreated consecutive patients (56 men, mean age 47.9 ± 8.8 yrs, range 28-67, and 44 women, mean age 53.5 ± 9.5 yrs, range 29-74) with a diagnosis of chronic hepatitis C virus infection who were scheduled for their first liver biopsy and naïve for antiviral therapy were prospectively included in this study. A peripheral blood sample was taken on the same day of the liver biopsy to determine cystatin C, creatinine and standard laboratory tests. Patients with serum creatinine > 1.20 mg/dL, HIV or active HBV infection, history of ethanol abuse (> 50 g of ethanol a day) or with primary hepatocellular carcinoma were excluded from the study.
A second group composed of 16 patients (9 men, mean age 62.3 ± 9.6) with a diagnosis of compensated (Child A) liver cirrhosis based on ultrasonographic and/or endoscopic evidence of portal hypertension, who fulfilled the remaining inclusion criteria, was included to evaluate the levels of serum cystatin C levels in more advanced stages of HCV-induced liver disease.
The study did not include the performance of procedures not justified by the clinical background of the patients. However, previous informed consent was obtained as a prerequisite for the inclusion. The study protocol conforms to the ethical guide lines of the 1975 Declaration of Helsinki and was approved by the local Ethics Committee (CEIC) of the Hospi-tal Clínico San Carlos.
Laboratory methodsSerum cystatin C was measured with the N Latex Cystatin C kit, a particle-enhanced immunone-phelometric method, on a BN ProSpec System (Siemens Healthcare Diagnostics). Serum creatinine was measured by means of the modified kinetic Jaffé method using a Beckman Coulter AU 5400 (Beckman Coulter).
Liver biopsy specimens obtained from patients of group 1 were examined by the same pathologist. Necroinflammation grade and fibrosis stage were scored according with the METAVIR system.10
Statistical analysisContinuous variables, expressed as mean (SD), were compared with the Student, t test or the MannWhitney U test, each when adequate, depending on their Gaussian distribution. A p value < 0.05 was considered significant. Categorical variables were compared with the x2 or the Fisher exact tests, each when appropriate, and the effect of differences was established by calculating the odds ratio with the 95 % confidence interval.
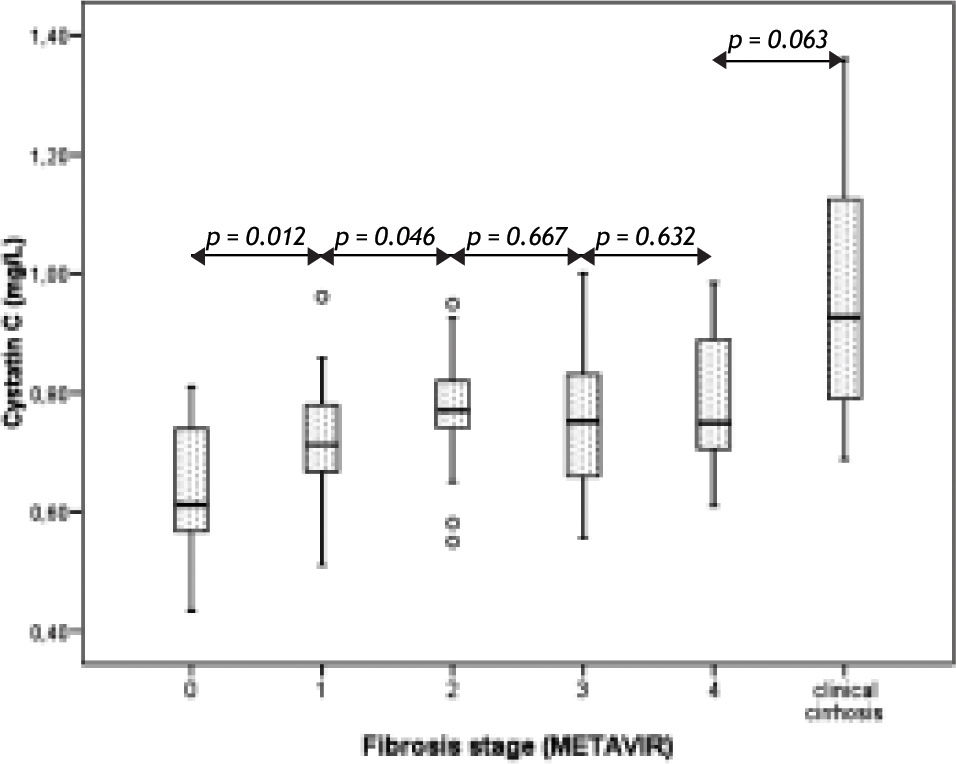
ResultsTable 1 reflects demographic and analytical variables in patients according their METAVIR fibrosis stage. As expected, a progressive decrease in platelet count and a progressive increase of the APRI score11 were observed with advancing fibrosis. Serum cystatin C levels also steadily increased with advancing METAVIR fibrosis stage, but only from stage 0 to stage 2, with a non-significant decline in stage 3 that plateau in stage 4 (Figure 1). However, cystatin C levels increased again in the group of non-biopsied cirrhotic patients (right column of table 1 and right bar of Figure 1).
Analytical variables* of patients according to METAVIR fibrosis stage.
| METAVIR stage | 0 | 1 | 2 | 3 | 4 | Compensated non-biopsied cirrhosis** |
|---|---|---|---|---|---|---|
| Number of patients | 16 | 35 | 21 | 17 | 11 | 16 |
| Age (years) | 47.4 (9.8) | 49.6 (9.3) | 48.4 (9.5) | 55.3 (8.7) | 53.4 (9.1) | 62.3 (9.6) |
| Sex (men/women) | 5/11 | 22/13 | 16/5 | 7/10 | 6/5 | 9/7 |
| Cystatin C (mg/L) | 0.64 (0.11) | 0.72 (0.09) | 0.78 (0.10) | 0.76 (0.14) | 0.78 (0.13) | 0.97 (0.20) |
| Creatinine (mg/dL) | 0.88 (0.14) | 0.92 (0.13) | 0.97 (0.14) | 0.89 (0.16) | 0.89 (0.08) | 0.93 (0.14) |
| Platelet (109/L) | 251 (59) | 219 (50) | 211 (60) | 194 (68) | 153 (44) | 119 (34) |
| AST (IU/L) | 36 (21) | 51 (28) | 54 (25) | 56 (39) | 74 (49) | 94 (59) |
| ALT (IU/L) | 58 (56) | 73 (54) | 87 (53) | 71 852) | 102 (63) | 89 (56) |
| AST/ALT | 0.76 (0.26) | 0,79 (0.28) | 0.68 (0.20) | 0.83 (0.17) | 0.77 (0.27) | 1.13 (0.39) |
| GGT (IU/L) | 29 (15) | 71 (71) | 91 (59) | 68 (51) | 73 (58) | 77 (48) |
| Cholesterol (mg/dL) | 184 (28) | 175 (31) | 170 (31) | 173 (38) | 169 (34) | 150 (32) |
| Ferritin (ng/mL) | 156 (217) | 187 (143) | 290 (261) | 175 (44) | 264 (175) | 151 (200) |
| APRI score | 0.37 (0.23) | 0.60 (0.33) | 0.72 (0.42) | 0.85 (0.90) | 1.31 (0.98) | 2.17 (1.60) |
As there were only 2 patients classified as METAVIR grade 0, grades 0 and 1 were included in the same group for comparisons. Serum cystatin C levels were significantly lower in patients with null-low necroinflammatory activity than in patients with more advanced grades (Table 2).
Plasma cystatin C levels in the 100 patients with chronic hepatitis C and liver biopsy classified according to METAVIR necroinflammatory grade.
| METAVIR necroinflammatory grade | Plasma cystatin C (mg/dL) | p value as compared with grade 0-1 | |
|---|---|---|---|
| Grade | Number of cases | ||
| 0-1 | 29 (2 + 27) | 0.69 (0.12) | - |
| 2 | 40 | 0.75 (0.11) | 0.047 |
| 3 | 31 | 0.75 (0.12) | 0.046 |
Our results show that serum cystatin C levels steadily increase along the earlier and intermediate stages of liver fibrosis in chronic hepatitis C, but this relation is lost when fibrosis reach the precirrhotic METAVIR stage.
The progression of fibrosis in chronic liver diseases results from an imbalance between synthesis and degradation of extracellular matrix. The synthesis depends on the activity of activated hepatic stellate cells and the degradation is carried out by a series of serinproteases, cysteine proteases and metalloproteinases.12 Cystatin C, a member of the cystatin family, is a strong inhibitor of lisosomal cysteine proteases that may act as a potent profibrogenic agent.4
Transforming growth factor ß (TGFß) is the most important cytokine inducing liver fibrogenesis, as it promotes the differentiation of hepatic stellate cells (HSC) in hepatic myofibroblasts. In vitro, TGFß is a potent inducer of Cys C secretion in vascular smooth muscle cells.13 Cys C expression is upregulated in the course of HSC transdifferentiation in myofibroblasts under the stimulus of TGFß.14
Plasma TGFß levels increase significantly with the severity of fibrosis in chronic hepatitis C, but declines when reaching the cirrhotic stage.15 As TGFß is the main stimulus for the production of cystatin C, this late decline may explain our finding of a decline in serum cystatin C levels in advanced liver fibrosis. Should it be interesting to simultaneously measure the serum levels of TGFß and cystatin C in chronic hepatitis C patients to confirm this hypothesis.
Hence, we suggest that serum cystatin C levels directly reflect the synthesis of TGFß in chronic hepatitis C and that they may be considered as a marker of ongoing fibrogenesis, but not as a marker of the fibrosis stage, as suggested preliminary studies that did not discriminate between the different stages of liver fibrosis induced by an heterogeneous group of liver noxae.8,9 In fact, if we had compared serum cystatin C levels in non-cirrhotic (89 patients) vs. all cirrhotic patients included in this study (27 cases) we had apparently confirmed previous findings suggesting that serum cystatin C level is useful as a non-invasive marker of liver fibrosis in chronic hepatitis C virus infection [0.73 (0.12) mg/L vs. 0.90 (0.20) mg/L, p < 0.001].
A drawback of this study is the reduced number of patients with advanced fibrosis in the liver biopsy. Liver biopsy is an invasive procedure and its practice is not allowed when the presence of cirrhosis in patients with chronic hepatitis C may be confirmed by non-invasive methods, as transient elastography or, in more advanced cases, by the presence of ultrasonographic or endoscopic evidence of portal hypertension. In our non-biopsied cirrhotic patients with more advanced, although still compensated, cirrhosis we have observed a second increase of serum cystatin C concentrations. As serum cystatin C has shown to be more sensitive for the assessment of renal function in patients with cirrhosis than creatinine-based criteria7,16 this increase could reflect an early reduction of glomerular filtration rate still not detected by plasma creatinine levels.
A second finding of our study is that serum cystatin C level is directly related to the necroinflammatory grade in chronic hepatitis C. Inflammatory signaling is the main stimulus for the hepatic stellate cells activation12 and again this correlation may be explained through the TGFß-dependent stimulus for Cys C production.
We conclude that serum cystatin C level may indirectly reflect current liver fibrogenic activity in chronic hepatitis C but it can not be considered as a non-invasive marker of liver fibrosis.
Conflict of InterestNone.