Background. VCAM-1 (soluble vascular cell adhesion molecule-1) plays a role in liver angiogenesis. Hepatocellular carcinoma (HCC) has important angiogenic activity, so expression of VCAM-1 may be pathogenic. Aim. To assess the association between serum VCAM-1 (sVCAM-1) levels and features of tumour and liver disease in patients with and without HCC, and to study the influence of HCC treatment on sVCAM-1 levels.
Material and methods. Concentrations in peripheral (sVCAM-1-P) and hepatic (sVCAM-1-H) veins were analysed using ELISA in 134 consecutive patients with chronic liver disease between May 2004 and February 2006, who underwent a splanchnic haemodynamic study. Of these patients, 58 had HCC.
Results. sVCAM-1-P and sVCAM-1-H were well correlated in both groups. No association was found between sVCAM-1-H and tumour features. No differences were observed in sVCAM-1-H between HCC and non-HCC cirrhotic patients. There was a significant linear association between Child-Pugh stage and sVCAM-1-H in HCC-patients (Child-Pugh A [2,485 ± 1,294 ng/mL] vs. Child-Pugh B [3,408 ± 1,338 ng/mL] vs. Child-Pugh C [4,096 ± 862 ng/ mL]; p = 0.007). Seven non-cirrhotic HCC patients had a significantly lower sVCAM-1-H than cirrhotic HCC patients. Treatment of HCC leads to an increase in sVCAM-1-H levels although this was not associated with the necrosis response to treatment.
Conclusions. sVCAM-1 levels are more closely associated with the severity of underlying liver disease than with the presence of HCC. sVCAM-1 levels are not associated with tumour features or invasiveness; therefore, sVCAM-1 does not seem to play an important role in the angiogenic processes of HCC.
Soluble vascular cell adhesion molecule-1 (VCAM-1), a member of the immunoglobulin superfamily, is expressed on various types of cells, including endothelial cells. This form is released into the bloodstream following proteolytic cleavage by vascular endothelial activation mediated by proinflammatory cytokines.1,2 This molecule plays an important role in providing attachment to the developing endothelium during angiogenesis.3,4 thus promoting adhesion of leucocytes to the endothelium.
Due to its angiogenic potency, elevated serum levels of VCAM-1 have been described in patients with gastric cancer,5,6 colorectal cancer,7 melanoma,8 leukaemia9 and breast cancer.10 VCAM-1 has been significantly correlated with tumour stage and the development of metastasis in neoplasms such as those presented above.
Up-regulated expression of VCAM-1 has also been reported in chronic liver disease, suggesting that VCAM-1 may play a role in the pathogenesis of chronic hepatitis and cirrhosis. Serum levels of this molecule (sVCAM-1) have been associated with increased fibrosis and angiogenesis in different aetiologies of liver disease.11–13 Furthermore, sVCAM-1 has recently been observed to act as a potential marker of hyperdynamic circulation, which is closely related to the different stages of liver cirrhosis.14
However, information about the influence of sVCAM-1 in patients with hepatocellular carcinoma (HCC) is scarce.15,16 Chronic hepatitis and cirrhosis are the underlying liver conditions in most patients with HCC; therefore sVCAM-1 could be involved in the progression of both the tumour and chronic liver disease.
Owing to the fact that sVCAM-1 is associated with increasing fibrosis and angiogenesis in liver disease and its direct relation with tumour burden in several neoplastic diseases, we hypothesized that this molecule may act as a marker of advanced HCC in cirrhotic patients.
Therefore, this study was designed to assess the correlation between sVCAM-1 levels and the clinical and tumour features of patients with HCC. We also evaluated the influence of locoregional treatment of HCC on sVCAM-1 levels and the response to treatment.
Material and MethodsWe performed a prospective single-centre cohort study between May 2004 and February 2006 in which 58 consecutive patients with HCC underwent a hepatic haemodynamic study to assess the hepatic venous pressure gradient (HVPG) as a prognostic criterion in candidates for treatment of HCC. Ten of these patients proceeded from a previous published cohort designed to assess the correlation between sVCAM-1 and splanchnic and systemic haemodynamic measurements.14 Due to the difficult for the performance of an analysis of association between sVCAM-1 with HCC features due to the small sample size in that previous study, we collected for the present study 48 patients with HCC between October 2004 and the final date of inclusion, in February 2006. In this period of time, 25 patients were evaluated for liver transplantation, and 20 finally received an organ. Seven patients underwent surgical resection of their tumour, 11 patients underwent radio-frequency ablation and 8 patients underwent transarterial chemoembolisation (TACE). Seven patients were treated with the best supportive care due to severe comorbidity or impaired liver function in cases with extensive HCC (in these cases the patients received a hepatic haemodynamic study in the context of a clinical trial). A further 76 consecutive patients analysed in the previously published cohort with cirrhosis but no radiological evidence of HCC and normal alpha-fetoprotein serve as comparative group.14 They also underwent a hepatic haemodynamic study as part of the routine clinical work-up. Patients with a previous liver transplant, HIV infection, or aetiology of liver disease due to Budd-Chiari syndrome or sinusoidal obstruction syndrome were excluded. The diagnosis of cirrhosis was established by liver biopsy or by a combination of clinical, biological and ultrasound findings. No patient had ongoing bacterial infection at the time of the haemodynamic measurements.
Demographic data and data on the aetiology of liver disease were collected. Biochemical and clinical variables were recorded to determine the model for end-stage liver disease (MELD) score17 and Child-Pugh score.18 The maximum permissible interval between the haemodynamic study and blood tests was 1 week; the maximum interval since the last dynamic imaging technique (computed tomography scan or magnetic resonance) was 1 month.
Diagnosis of HCC was established following the algorithm of the American Association for the Study of Liver Diseases (AASLD).19 The Barcelona Clinic Liver Cancer (BCLC) classification was used to stage HCC.20
Treatment performance and definition of responseOf the whole HCC cohort, 8 patients underwent TACE and 11 patients underwent radiofrequency ablation. Of these, sVCAM-1-P levels were determined in 9 patients treated with radiofrequency ablation and in 5 patients treated with TACE, at days 1, 3 and 7 after treatment was performed. The selection of these patients was due to the inclusion of them in another prospective study.
Radiofrequency ablation was performed percutaneously except when the nodule was in a difficult access location or in the proximity of other organs or blood vessels, performing in thoses cases by laparos-copy or laparotomy accesses. The electrode used for performing the procedure was a Berchtold Medical Electronics with an Elektrotom 106 HF-Thermo generator (Berchtold Medizin-ElektroniK Tuttlingen) at 350 KHz, with saline solution perfussion of 60–80 mL/h, and with a temperature threshold of 70 oC to 100 oC.
TACE was performed using a standard mixture of 60 mg of adriamicin with 12 cc of lipiodol, or in case of lesions with less size the mixture consists in 40 mg of adriamicin with 8 cc of lipiodol. After the delibery of the mixture the vessels were embolized with gelfoam.
The response to the treatment was evaluated 6 weeks after treatment performance by dynamic imaging techniques. All the imaging files were reviewed subsequently for this purpose in the present study, using the modified Response Evaluation Criteria in Solid Tumors (RECIST) for HCC.21 Complete Response was considered in the case of disappearance of any intratumoral arterial enhancement in all target lesions after treatment. Fail of treatment was considered in the rest of cases.
Haemodynamic measurementsAfter an overnight fast, the patient was prepared for the study in the supine position. Under local anaesthesia, a vascular introducer sheath (Medikit Co Ltd, Tokyo, Japan) was placed into the right internal jugular vein. Then, under fluoroscopy, a 7F balloon catheter (Cordis SA, Miami, FL, USA) was conducted to the right hepatic vein for the measurement of free and wedged hepatic venous pressure (FHVP and WHVP) as described previously.22 FHVP was measured in the hepatic vein with the tip of the catheter just beyond the junction with the inferior vena cava. HVPG was calculated as WHVP minus FHVP. Portal hypertension was defined as the presence of an HVPG > 5 mmHg. All haemodynamic measurements were performed using a previously calibrated strain-gauge transducer and recorded at least in duplicate.
The tracings of the haemodynamic studies were evaluated by two independent investigators who were unaware of the identity and diagnosis of the patient.
Laboratory determinationsBlood samples were collected from peripheral and hepatic veins during the haemodynamic studies. sVCAM-1 levels were quantified using an enzyme-linked immunosorbent assay (ELISA) kit designed to measure human soluble VCAM-1 concentration in serum (Human VCAM-1 Immunoassay, R&D System Minneapolis, MN, USA). sVCAM was detected by a monoclonal murine antibody to human VCAM-1. Each measurement was made in duplicate and serum VCAM-1 levels were determined by extrapolation from a standard curve generated for each set of samples assayed. The test has a sensitivity threshold of < 2.0 ng/mL, an intra-assay variability of 4.3%, an interassay variability of 8.5%, and a mean reference value of 553 ng/mL (range 395-714 ng/mL) on a panel of 105 blood donors.
Serum hyaluronic acid and serum vascular endothelial growth factor (VEGF) levels were measured by ELISA according to the manufacturer’s instructions (Corgenix Inc., Denver, Colorado, USA and R&D System Minneapolis, Minnesota, USA).
Statistical analysisQuantitative variables were expressed as the mean (Standard Deviation) or median (Interquartile Range), and qualitative variables were expressed as frequencies. Categorical variables were compared using the χ2; continuous variables were compared using the t test or the Mann-Whitney U test when appropriate. One-way analysis of variance (ANOVA) with polynomial contrasts was applied. Pearson or Spearman correlations were used to evaluate the association between continuous variables as appropriate. In the case of performing multiple comparations between variables the Bonferroni correction was applied as appropiate. The differences in the mean values of sVCAM after treatment performance were evaluated by a general linear model comparing the variables by the Bonferroni method. Those variables with a p value of 0.1 or less were chosen for enter in a multivariate analysis with a multiple linear regression model. Statistical significance was set at a p value of 0.05 (2-tailed). Statistical analysis was performed using SPSS 15.0 (SPSS® 15.0; SPSS Inc., Chicago, Illinois, USA).
Ethical considerationsThe study protocol was approved by the local Ethics Committee and the Institutional Review Board, and was in accordance with the Helsinki Declaration. All the samples were collected after a signed informed consent was obtained from each patient before the hepatic haemodynamic study.
ResultsPatient populationThe clinical characteristics of the HCC and non-HCC population are shown in table 1. In the HCC patients, the median age was 60 (52–70) years, and 81% were men. The main aetiologies of liver disease were hepatitis C virus infection (69%) and alcohol abuse (15.5%). Five patients were infected by hepatitis B virus and all but one of them had data of cirrhosis. One of these patients was received antiviral treatment with lamivudin and two with lamivudin and adefovir. Most patients were Child-Pugh grade A (62.1%). Compared with the non-HCC patients, patients with HCC were older, showed a better MELD score and liver function parameters, and had less episodes of hepatic decompensation. Seven patients with HCC did not have established cirrhosis as their underlying liver disease.
Epidemiological and clinical characteristics of patients with and without hepatocellular carcinoma.
| HCC patients (n = 58) | Non-HCC patients (n = 76) | p | |
|---|---|---|---|
| N (%) | |||
| Sex (Male/Female) | 47 (81)/11 (19) | 53 (70) / 23 (30) | 0.13 |
| Aetiology | 0.25 | ||
| Hepatitis C virus | 40 (69) | 42 (55) | |
| Alcohol | 9 (15) | 31 (41) | |
| Hepatitis B virus | 5 (9) | 3 (4) | |
| Other | 4 (7) | - | |
| Cirrhosis criteria fulfilled | 51 (88) | 76 (100) | 0.002 |
| Child-Pugh (A/B/C) | 36 (62) / 16 (28) / 6 (10) | 26 (34) / 37 (49) / 13 (17) | 0.005 |
| Ascites | 16 (28) | 39 (51) | 0.007 |
| Hepatic encephalopathy | 2 (4) | 4 (5) | 0.99 |
| Presence of oesophageal varices | 32 (60) | 61 (80) | 0.007 |
| Previous variceal bleeding | 5 (9) | 13 (17) | 0.17 |
| Mean ± SD | |||
| Age (years) | 60.3 ±11.5 | 50.3 ± 7.6 | 0.001 |
| Platelets (cells/μL) | 131,569 ± 89,646 | 100,921 ± 60,713 | 0.03 |
| INR | 1.19 ± 0.38 | 1.51 ±0.55 | 0.001 |
| Bilirubin (mg/dL) | 1.9±1.6 | 3.9 ±4.9 | 0.003 |
| Albumin (g/dL) | 3.6 ±0.6 | 3.2 ±0.6 | 0.001 |
| Creatinine (mg/dL) | 1 ± 0.7 | 0.9 ±0.7 | 0.66 |
| MELD score | 10±5 | 15±6 | 0.001 |
INR: international normalized ratio. MELD: model for end stage liver disease.
Tumour characteristics are shown in table 2. BCLC stage A was predominant (55.2%), mean tumour size was 4.8 ± 3.7 cm, the lesion was solitary in 63.2% of patients, both lobes were involved in 19.2% of cases, and vascular invasion was present in 6.9% of patients.
Correlation of sVCAM-1 between peripheral and hepatic veinsA strong correlation was observed in levels of sVCAM-1 between samples collected from peripheral veins (sVCAM-1-P) and those from hepatic veins (sVCAM-1-H) in the whole cohort (r = 0.80; p = 0.0001) (Figure 1). This correlation was also very high in patients with HCC (r = 0.85; p = 0.0001) and without HCC (r = 0.77; p = 0.0001).
Association between sVCAM-1-H and features of HCC
Table 2 shows the relationship between sVCAM-1-H levels and the features of HCC. An almost significant statistically inverse correlation was found between sVCAM-1-H concentration and total tumour size in centimetres in both Child-Pugh A and B-C patients (p = 0.08). There was no significant association in either Child-Pugh group with the following: alpha-fetoprotein levels, lobular tumour extension, vascular thrombosis, lymph node involvement, fulfilment of the Milan criteria, number of nodules or infiltrating tumour pattern. The differences with respect to BCLC stage were significant throughout the HCC cohort, although these differences were lost when we performed a subgroup analysis based on Child-Pugh stage.
Tumour characteristics of HCC patients and association with sVCAM-1-H levels.
| N (%) | Mean ± SD sVCAM-1-H (ng/mL) | p | |
|---|---|---|---|
| Total tumour size | 0.08 | ||
| < 3 cm | 22 (38) | 3,424 ± 1,477 | |
| 3–5 cm | 16 (28) | 2,407 ± 1,153 | |
| > 5 cm | 20 (34) | 2,704 ± 1,314 | |
| Number of nodules† | 0.23 | ||
| Single nodule | 37 (69) | 2,989 ± 1,455 | |
| Multinodular | 17 (31) | 2,476 ± 160 | |
| Infiltrating pattern | 0.11 | ||
| Present | 4 (7) | 3,991 ± 814 | |
| Absent | 54 (93) | 2,835 ± 1,382 | |
| Lobar tumour extension | 0.88 | ||
| One lobe | 46 (79) | 2,936 ± 1,446 | |
| Bilobar | 12 (21) | 2,865 ± 1,156 | |
| Vascular thrombosis | 0.24 | ||
| Present | 4 (7) | 3,700 ± 1,069 | |
| Absent | 54 (93) | 2,858 ± 1,389 | |
| Lymph node involvement | 0.91 | ||
| Present | 17 (29) | 2,951 ± 1,357 | |
| Absent | 41 (71) | 2,908 ± 1,407 | |
| Milan criteria fulfilled | 0.16 | ||
| Yes | 42 (72) | 3,089 ± 1,355 | |
| No | 16 (28) | 2,521 ± 1,390 | |
| Alphafetoprotein > 20 ng/mL | 0.36 | ||
| Yes | 32 (55) | 2,760 ± 1,309 | |
| No | 26 (45) | 3,107 ± 1,457 | |
| Alpha-fetoprotein > 200 ng/mL | 0.38 | ||
| Yes | 50 (86) | 2,851 ± 1,387 | |
| No | 8 (14) | 3,321 ± 1,335 | |
| BCLC stage‡ | 0.02 | ||
| 0 very early | 8 (14) | 2,818 ± 1,273 | |
| A early | 32 (55) | 3,005 ± 1,364 | |
| B intermediate | 7 (12) | 1,577 ± 587 | |
| C advanced | 5 (9) | 3,042 ± 1,703 | |
| D terminal | 6 (10) | 4,097 ± 862 |
BCLC: Barcelona Clinic Liver Cancer.
In addition to this, a model of linear regression was performed adjusting the model with the BCLC, observing that this variable only explained the 6.7% of the variability of sVCAM-1-H with absence of statistical significance (p = 0.059) (Table 3). Therefore, the influence of liver function impairment was predominant.
Correlation of continuous variables with sVCAM-1-H in HCC patients.
| sVCAM-1-H (ng/mL) | ||
|---|---|---|
| r | p | |
| sVCAM-1-P (ng/mL) | 0.85 | 0.0001 |
| MELD score | 0.27 | 0.048 |
| Bilirubin (mg/dL) | 0.47 | 0.0001 |
| HVPG (mmHg) | 0.38 | 0.007 |
| Albumin (g/dL) | −0.45 | 0.001 |
| Platelets (cells/µL) | −0.49 | 0.0001 |
| Leucocites (cells/µL) | −0.38 | 0.005 |
| Haemoglobin (g/dL) | −0.22 | 0.1 |
| AST (U/L) | 0.41 | 0.026 |
| GGT (U/L) | −0.03 | 0.81 |
| Hyaluronic acid (ng/mL) | 0.7 | 0.0001 |
| VEGF (pg/mL) | −0.36 | 0.06 |
| Alpha-fetoprotein (ng/mL) | 0.06 | 0.68 |
| INR | 0.11 | 0.4 |
| Serum sodium (mEq/L) | 0.03 | 0.87 |
MELD: model for end stage liver disease. HVPG: hepatic venous pressure gradient. AST: aspartate aminotransferase. GGT: gamma-glutamil transpeptidase. VEGF: vascular endothelium growth factor. INR: international normalized ratio.
In HCC patients, sVCAM-1-H levels had no significant relationship with sex, age, aetiology or previous alcohol consumption. There was a significant linear association between Child-Pugh stage and sV-CAM-1-H (Child-Pugh A [2,485 ± 1,294 ng/mL] vs. Child-Pugh B [3,408 ± 1,338 ng/mL] vs. Child-Pugh C [4,096 ± 862 ng/mL]; p = 0.007) (Figure 2A), as well as a significant positive correlation with the MELD score (r = 0.27) (Figure 2B), total serum bilirubin (r = 0.47) and HVPG (r = 0.38). A significant inverse correlation was observed with serum albumin level (r = -0.45), platelet count (r = −0.49), and white cell count (r = −0.38). No association was demonstrated with the level of AST. These results were comparable in the entire cohort of HCC and non-HCC patients. The correlations between sVCAM-1-H and continuous variables are expressed in table 3.
Association between sVCAM-1-H levels and different features in HCC patients. A. Levels of sVCAM-1-H (ng/mL) according to the Child-Pugh classification. B. Levels of sVCAM-1-H (ng/mL) according to the model for end-stage liver disease (MELD) score. C. Correlation of sVCAM-1-H levels (ng/mL) with hyaluronic acid concentration (ng/mL). sVCAM-1-H: soluble vascular cell adhesion molecule-1 in hepatic veins.
Although without reaching significant association, in the HCC group the relationship between sVCAM-1-H and complications of cirrhosis demonstrated higher levels of this molecule in patients with previous history of hepatic decompensation: ascites (3,092 ± 958 vs. 2,854 ± 1,534 ng/mL), presence of esophageal varices (3,272 ± 1,302 vs. 2,719 ± 1,398 ng/mL) or variceal bleeding (3,444 ± 1,584 vs. 2,867 ± 1,391 ng/mL). In agreement with these data, sVCAM-1-H levels were different among the clinical stages proposed in the Baveno IV consensus conference, showing an increase in the levels compared with patients without decompensation (stage 1: 2,489 ± 1,495 vs. stage 2: 3,493 ± 1,432 vs. stage 3: 3080 ± 970 vs. stage 4: 2,552 ± 1,387; p = 0.16).
A multivariate linear regression model was performed with the entire cohort to investigate the influence of sVCAM-1-H in the severity of liver disease. Finally the model contained the following variables: serum bilirubin, MELD score, HVPG and Baveno IV stages (1-2-3 vs. 4), explaining the 42.8% of the variability of the influence of sVCAM-1-H in this setting, with a high significance (p < 0.001) (Table 4).
Multivariate linear regression models for tumoral features and severity of liver disease explaining sVCAM-1-H.
| R | R2 | B (SE) | Inferior CI95% | Superior CI95% | P | |
|---|---|---|---|---|---|---|
| Model for tumoral features | ||||||
| BCLC (A-B vs. C-D) | 0.258 | 0.067 | −875.87 (453.87) | −1,785.94 | 35.57 | 0.059 |
| Model for severity of liver disease features | ||||||
| Final model | 0.654 | 0.428 | 2,052.15 (463.35) | 1,117.72 | 2,986.58 | < 0.001 |
| Bilirubin | - | - | 932.08 (226.14) | 476.03 | 1,388.12 | < 0.001 |
| MELD | - | - | −143.13 (56.88) | −257.84 | −28.43 | 0.016 |
| HVPG | - | - | 53.72 (26.97) | −0.68 | 108.11 | 0.053 |
| Baveno IV (1-2-3 vs. 4) | - | - | −1,365.63 (696.69) | −2,770.64 | 39.37 | 0.056 |
BCLC: Barcelona Clinic Liver Cancer. MELD: model for end stage liver disease. HVPG: hepatic venous pressure gradient.
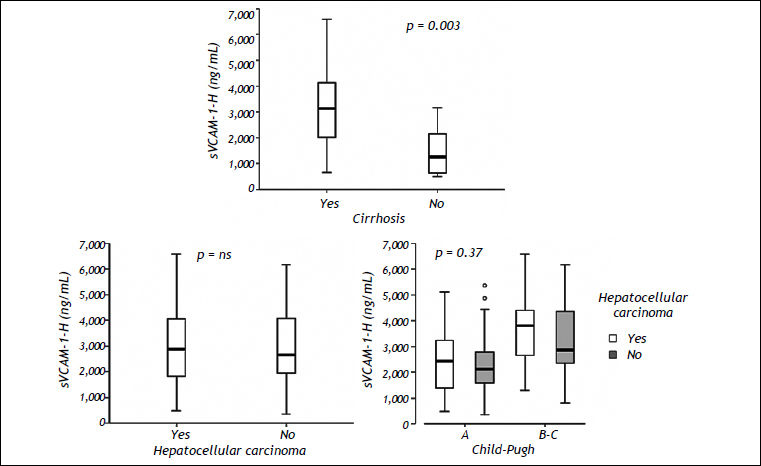
Seven HCC patients had no diagnosis of cirrhosis. The mean level of sVCAM-1-H in these patients was 1,460 ± 1,030 ng/mL, which was significantly lower than that observed in the HCC patients with cirrhosis (3,099 ± 1,301 ng/mL; p = 0.003) (Figure 3).
Association between sVCAM-1-H levels and the severity of liver disease. A. Differences in sVCAM-1-H levels (ng/mL) between HCC patients with and without cirrhosis. B. Association between sVCAM-1-H levels (ng/mL) and the presence of hepatocellular carcinoma in the whole cohort. C. Levels of sVCAM-1-H (ng/mL) according to the Child-Pugh classification in patients with and without HCC. sVCAM-1-H: soluble vascular cell adhesion molecule-1 in hepatic veins.
Serum levels of hyaluronic acid in peripheral and hepatic veins were measured in 21 patients as a marker of liver fibrosis. sVCAM-1-H was highly correlated with both measurements (r = 0.72 and r = 0.70, respectively; p = 0.0001) (Figure 2C).
Furthermore, levels of VEGF were analysed in the peripheral and hepatic veins of 28 patients, and no robust association was found with sVCAM-1-H levels (r = -0.33 and r = -0.36, respectively; p = 0.06).
Association between sVCAM-1-H and cirrhotic patients with and without HCCThe distribution of sVCAM-1-H levels in cirrhotic and non-cirrhotic patients with and without HCC is shown in figure 3. The 7 non-cirrhotic patients with HCC had a significantly lower level of sVCAM-1-H than cirrhotic patients (1,460 ± 1,030 vs. 2,900 ± 1,424 ng/mL; p = 0.003) (Figure 3A).
The mean sVCAM-1-H levels were 3,099 ± 1,301 ng/mL in the HCC group and 2,900 ± 1,424 ng/mL in the non-HCC group with no significant differences between the groups (Figure 3B). These results were maintained when we analysed the differences between both groups stratified by Child-Pugh stage (A vs. B-C) (Figure 3C) or MELD score (MELD > 15 vs. < 15).
In the follow-up 8 patients without HCC in the moment of inclusion in the study, developed HCC. The median time for the detection of the tumor after the performance oh the basal haemodynamic study was 11 (3.25–30.75) months. Three of these patients received a liver transplant in the follow-up because of HCC. Other two patients without HCC in the inclusion time who were transplanted in the follow-up, developed an HCC that was discovered incidentally in the liver explanted, without correlation with the previous dynamic imaging techniques. There were no differences in sVCAM-1-H concentration between the patients who developed (8/76) and not developed the tumor (2,595 ± 1,248 vs. 2,976 ± 1,446 ng/ mL; p = 0.48).
HCC treatment and sVCAM-1 concentrationWe measured sVCAM-1-P levels in 14 patients who received treatment for HCC during follow-up, at days 1, 3 and 7, and 6 weeks after treatment was performed. Radiofrequency ablation was performed in 9 patients and TACE was chosen in 5 patients. Complete response was achieved in 22% of patients received radiofrequency ablation compared with 80% of those received TACE. The median number of sessions in the radiofrequency ablation group was 1.4. This could cause a suboptimal complete response in the Radiofrequency group. We observed a progressive increase in levels of sVCAM-1-P over time with a maximum level in the seventh day, but without statistically significant differences between the measures at 1st, 3rd or 7th day after the treatment performance. Levels then decreased to their baseline values (Figure 4). Although higher levels of sVCAM-1-P were associated with failure of achievement of complete response of HCC in both kind of treatments, this difference was not statistically significant (complete response 56.5% [2,985 ± 1,486 ng/mL] vs. non-complete response 43.5% [3,786 ± 1,498 ng/mL]; p = 0.21).
DiscussionAlthough the importance of VCAM-1 as a circulating angiogenesis-related marker and its role in the development and prognosis of several types of cancer are well established, its effect on HCC is uncertain and literature reports are scarce.15,16 One important difference between our study and previous reports is the measurement of VCAM-1 from hepatic and peripheral veins. This allowed us to determine VCAM-1 titers directly at the drainage site of those molecules released by the tumour and the non-cancerous liver tissue. In this sense we have demonstrated a significant correlation between both sites; therefore VCAM-1 maintains its levels once it has been released from intrahepatic circulation. This finding will make it easier to measure the “real” level of VCAM-1 in future studies. One advantage of the strong correlation observed for sVCAM-1 concentrations in the peripheral and hepatic veins was the opportunity to perform an analysis using sVCAM-1-H, because the most reliable levels were associated with VCAM-1 released directly by both the tumour and non-cancerous liver tissue. The results of the analysis using sVCAM-1-P are consistent with those shown for sVCAM-1-H in the present study.
It has been reported that sVCAM-1 is overexpressed in chronic liver disease and that overexpression closely reflects the presence of liver cirrhosis23,24 and portal hypertension.25
So, the presence of an underlying chronic liver disease in patients with HCC can make it difficult to analyse sVCAM-1 levels due to the fact that chronic hepatitis or cirrhosis in the non-cancerous liver tissue could also contribute to the release of VCAM-1. This is seen clearly in our study with the observation that high sVCAM-1 levels in HCC patients correlate positively with the MELD score and total serum bilirubin levels and inversely with platelet and white cell count and serum albumin levels, suggesting that sVCAM-1 levels are associated with the severity of underlying liver disease and progressive impairment of liver function. Likewise, sVCAM-1 levels increase with a poorer Child-Pugh grade in patients with HCC, and patients without cirrhosis have a significantly lower sVCAM-1 concentration.
Due to the differences in liver function between patients with and without HCC, we have performed our analysis based on Child-Pugh subgroups to avoid possible bias in the interpretation of the results. These differences are well explained because the most of the patients in the HCC group were received the haemodynamic study in the context of the evaluation for liver transplant and surgical resection of HCC in whom the measurement of HVPG is systematically performed. So, in the HCC group the 69% of patients have a BCLC stage of 0 or A, usually associated with a well preserved liver function.
In a recent study14 multivariate regression analysis showed sVCAM-1 to be associated with systemic vascular resistance in patients with cirrhosis, and patients who died or underwent transplantation during follow-up had significantly greater values of sVCAM-1 at baseline than those who did not. These findings may lead to a better understanding of fibrogenesis, and identifying related biological markers could prove helpful during clinical follow-up, when assessing response to current antiviral or antifibrotic therapy, and, in patients with end-stage liver disease, for prioritizing transplant candidates.
Serum hyaluronic acid is a well-known marker of liver fibrosis, and its levels are increased in patients with severe fibrosis or cirrhosis.26 In our study, sVCAM-1 levels are directly correlated with the concentration of serum hyaluronic acid, thus supporting the relationship between sVCAM-1 and the presence of cirrhosis in patients with HCC.
In our study, sVCAM-1 are not associated with the clinical features of HCC, contrary to the finding of higher sVCAM-1 levels associated with more advanced-stage tumours in other cancers. Indeed, sVCAM-1 is reduced in patients with larger tumours. These observations are consistent with those of Ho, et al.15 and Hyodo, et al.16
Our results show how the release of VCAM-1 in hepatic circulation seems to depend to a greater extent on the non-cancerous tissue than the tumour, since there are no significant differences between sVCAM-1 levels in patients with and without HCC, so these levels increase in line with the degree of liver function impairment. Although a small number of patients in each group could be criticized as a possible bias in this association, we think that the analysis with this cohort of patients is correct due to the little data about this aspect in the literature and the far apart of the result of significance.
The reason why expression of sVCAM-1 is down-regulated in patients with HCC is unknown, mostly due to the lack of data on this molecule in the context of HCC. In fact, the concentration of VEGF, an important pro-angiogenic molecule whose levels are high in many tumours,27–30 including HCC,31–33 is inversely correlated with sVCAM-1 levels in our study. Although there are no robust conclusions, this may lead to think that sVCAM-1 is not a basic angiogenic factor in the development of HCC. In this sense, using immunoperoxidase staining of VCAM-1 in tissue from patients with chronic hepatitis, cirrhosis and HCC, Hyodo, et al.16 demonstrated that VCAM-1 was distributed on the endothelial cells of the vessels and the dendritic cells at lymphocyte aggregations in the portal areas, and on Kupffer cells and sinusoidal endothelial cells in the liver tissue of patients with chronic hepatitis and cirrhosis, but that it was not expressed by HCC cells. Further studies analysing the implication of VCAM-1 in the angiogenic pathways of HCC and its correlation between cancerous liver tissue and serum may help clarify the real role of this molecule in HCC.
The influence of loco-regional treatment of HCC in the release of VCAM-1 and its subsequent serum measurement is not well known. Only one study has evaluated this premise but did not find changes in serum concentrations of VCAM-1 during the first 24 h after TACE.34 In our study, we observe an increase in sVCAM-1 levels over time, with a peak on the seventh day in response to tumour necrosis, but non-cancerous tissue necrosis may also have a significant influence on the release of VCAM-1. This increase in sVCAM-1 levels due to ischemic injury has been observed in patients after hepatectomy.35 The concentration of post-treatment sVCAM-1 was not associated with tumour response. Although the performance of survival analysis and disease free recurrence rate in our work could be provided interesting data, the heterogeneity of the treatments applied to the HCC patients and the small sample size of treated patients would made impossible to express a solid conclusion, so our study was not designed to evaluate survival, but Ho, et al.15 demonstrated that low preoperative serum levels of VCAM-1 in patients undergoing liver resection was directly associated with better disease-free survival and overall survival. Therefore, future studies must elucidate the potential influence of sVCAM-1 as a prognostic marker in patients with HCC who have undergone treatment.
On the other hand, although the results of the study are negatives to respect the influence of sVCAM-1 in HCC, we think that our results are important in that they allow to discard this molecule in future investigations about the development of new antiangiogenic drugs in the treatment of HCC.
In conclusion, our study demonstrates that release of sVCAM-1 arises more from the severity of underlying liver disease and the existence of cirrhosis, rather than from the presence of HCC. sVCAM-1 levels are not associated with the tumour or invasiveness features in HCC patients, and there are no differences with patients who do not have HCC; therefore, sVCAM-1 does not seem to play an important role in the angiogenic processes of HCC. The ischemic stroke caused by loco-regional treatment of HCC stimulates the release of VCAM-1, although this is not correlated with the response to treatment or necrosis. Further studies with a well-defined primary objective and adequate sample size are needed before drawing significant conclusions about this marker.
Abbreviations- •
VCAM-1: soluble cell adhesion molecule-1.
- •
HCC: hepatocellular carcinoma.
- •
sVCAM-1: serum soluble cell adhesion molecule-1.
- •
sVCAM-1-P: serum soluble cell adhesion molecule-1 in peripheral veins.
- •
sVCAM-1-H: serum soluble cell adhesion molecule-1 in hepatic veins.
- •
ELISA: enzyme-linked immunosorbent assay.
- •
HVPG: hepatic venous pressure gradient.
- •
TACE: transarterial chemoembolisation.
- •
MELD: model for end-stage liver disease.
- •
AASLD: American Association for the Study of Liver Diseases.
- •
BCLC: Barcelona Clinic Liver Cancer.
- •
RECIST: Response Evaluation Criteria in Solid Tumors.
- •
FHVP: free hepatic venous pressure.
- •
WHVP: wedged hepatic venous pressure.
- •
VEGF: vascular endothelial growth factor.
- •
INR: international normalized ratio.
All the authors disclosure no financial relationship that might lead to a conflict of interest in relation to the manuscript.
AcknowledgementsWe are indebted to Tom O’Boyle, Virginia Sebastian and Cristina Fernandez for their expert management in the English correction and statistical analysis of the present manuscript.