Treatment of chronic hepatitis B (CHB) with nucelos(t)ide analogues (NA) can improve outcomes, but NA treatment is expensive for insurance plans.
Materials and MethodsThe Centers for Medicare & Medicaid Services database was assessed from 2012 to 2021 to assess the use of NA for CHB in patients on Medicaid. Data extracted included the number of claims, units, and costs of each agent stratified by originator and generic.
ResultsOver the study period, 1.9 billion USD was spent on NA, with spending peaking in 2016 at $289 million US, which has subsequently decreased. Lower expenditures since 2016 have been associated with increased use of generics. The use of generic tenofovir or entecavir led to savings of $669 million US over the study period.
ConclusionsIncreased generic use has significantly reduced expenditures for NA drugs; policy shifts towards generic drug use may help with sustainability.
Approximately 1.6 million individuals live with chronic hepatitis B (CHB) in the United States (US) [1]. CHB is associated with an increased risk of developing cirrhosis and hepatocellular carcinoma, which can often be prevented by suppression of HBV viremia (i.e., viral load). Nucleoside and nucleotide analogues (NA) are used in the treatment of CHB, but NA therapy is often prolonged and expensive [2]. Medicaid is the largest source of health coverage in the US and provides coverage to pregnant individuals, individuals with disabilities and those with lower incomes [3]. The aim of this study is to assess the costs associated with CHB treatment in individuals eligible for Medicaid reimbursement through a cross-sectional study.
2Materials and MethodsData from the publicly available Centers for Medicare & Medicaid Services Medicaid database was accessed [4] from 2012-2021 to ascertain NA usage for treatment of CHB: lamivudine (LAM/Epivir-HBV®), adefovir (ADF/Hepsera®), entecavir (ETV/Baraclude®), tenofovir disoproxil fumarate (TDF/Viread®) and tenofovir alafenamide (TAF/Vemildy®). Data extracted included the number of units, claims and cost ($USD) of each agent, classified by originator and generic versions of each compound. The number of patients on treatment and the specific indications for the use of each NA is not available in this database. Costs are based on the gross price, which represents the total spending by all payers, including Medicaid. Rebates and discounts from manufacturers were not included in our analysis as this is proprietary information [4]. To estimate the cost savings realized by generic medication as compared to the originator, the difference between the average cost/unit for the originator and generic was determined and multiplied by the number of generic units prescribed. Microsoft Excel (v16.75) was used for data analysis and Joinpoint Regression Program (version 4.9.1.0, National Cancer Institute) was used to calculate annual percentage change (APC) and perform trends analysis. This study was unfunded.
2.1Ethical statementAs the utilized data is publicly accessible and devoid of identifiable information, formal ethics approval was deemed unnecessary.
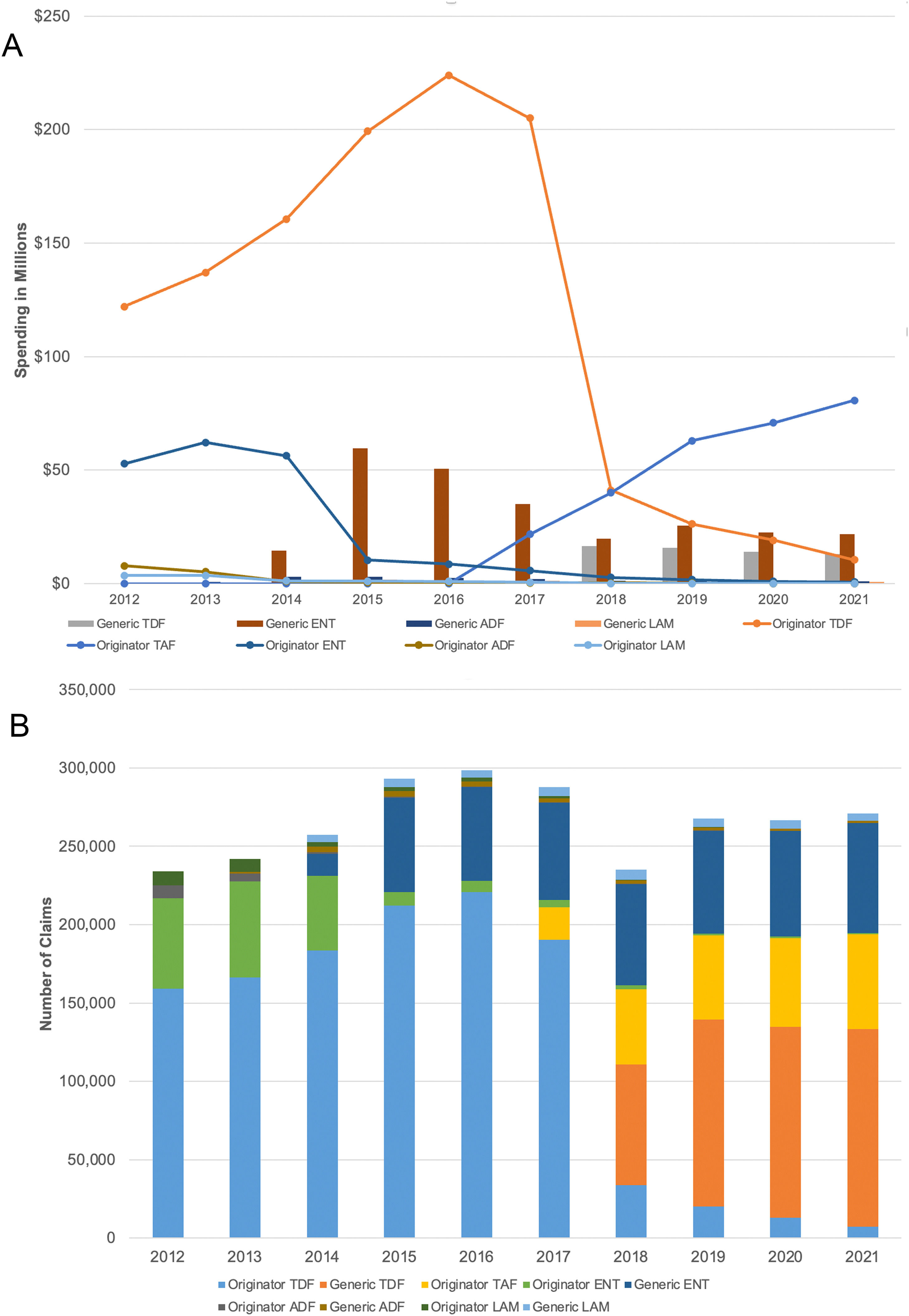
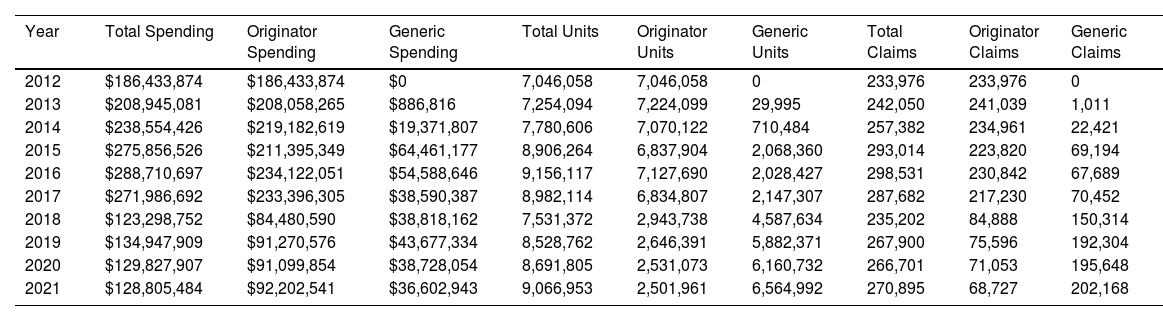
3ResultsFrom 2012 to 2021, the number of enrollees in Medicaid increased from 58.9 million in 2012 to 86.3 million in 2021 [5], with the total Medicaid spending on NA being 1.9 billion $USD dollars for 1,682,132 claims. The number of claims submitted increased from 233,976 in 2012 and reached a peak in 2016 before trending downwards to a plateau of about 270,000 (2012-2015: APC, 8.0; 95% CI 2.2-22.0; 2015-2021 APC, -1.7; 95% CI -0.9 to 0.4). Similarly, the spending for NAs grew on average, reaching a peak of $289 million in 2016, before decreasing to $129 million in 2021 (2012-2016: APC, 11.1%; 95% CI 0.5-39.90; 2016-2021: APC, -18.4; 95% CI -34.2 to -11.7). The reduced expenditure is due to decreased utilization of originator drugs (2015-2021: APC, -1.67, 95% CI -9.9 to 0.4) with a linked spending decrease (2016-2021: APC, -21.0; 95% CI -42.6 to -13.7) due to the introduction of generics (adefovir in 2013, lamivudine and entecavir in 2014, TDF in 2017) [1,6] (Table 1).
Medicaid spending, units and claims for nucleoside/nucleotide analogues from 2012-2021 in the US
| Year | Total Spending | Originator Spending | Generic Spending | Total Units | Originator Units | Generic Units | Total Claims | Originator Claims | Generic Claims |
|---|---|---|---|---|---|---|---|---|---|
| 2012 | $186,433,874 | $186,433,874 | $0 | 7,046,058 | 7,046,058 | 0 | 233,976 | 233,976 | 0 |
| 2013 | $208,945,081 | $208,058,265 | $886,816 | 7,254,094 | 7,224,099 | 29,995 | 242,050 | 241,039 | 1,011 |
| 2014 | $238,554,426 | $219,182,619 | $19,371,807 | 7,780,606 | 7,070,122 | 710,484 | 257,382 | 234,961 | 22,421 |
| 2015 | $275,856,526 | $211,395,349 | $64,461,177 | 8,906,264 | 6,837,904 | 2,068,360 | 293,014 | 223,820 | 69,194 |
| 2016 | $288,710,697 | $234,122,051 | $54,588,646 | 9,156,117 | 7,127,690 | 2,028,427 | 298,531 | 230,842 | 67,689 |
| 2017 | $271,986,692 | $233,396,305 | $38,590,387 | 8,982,114 | 6,834,807 | 2,147,307 | 287,682 | 217,230 | 70,452 |
| 2018 | $123,298,752 | $84,480,590 | $38,818,162 | 7,531,372 | 2,943,738 | 4,587,634 | 235,202 | 84,888 | 150,314 |
| 2019 | $134,947,909 | $91,270,576 | $43,677,334 | 8,528,762 | 2,646,391 | 5,882,371 | 267,900 | 75,596 | 192,304 |
| 2020 | $129,827,907 | $91,099,854 | $38,728,054 | 8,691,805 | 2,531,073 | 6,160,732 | 266,701 | 71,053 | 195,648 |
| 2021 | $128,805,484 | $92,202,541 | $36,602,943 | 9,066,953 | 2,501,961 | 6,564,992 | 270,895 | 68,727 | 202,168 |
Tenofovir based therapy (TDF, TAF) made up 74.5% of the total expenditure from 2012-2021 (TDF-originator 57.6%, TAF-originator 13.9%), entecavir 22.76% (originator 10.2%) with only a minority of expenditures due to adefovir (1.65%) and lamivudine (1.07%) (Fig. 1). Notably, TAF introduced in 2017, currently reflects 63% of the 2021 expenditure on NA and 22% of the claims.
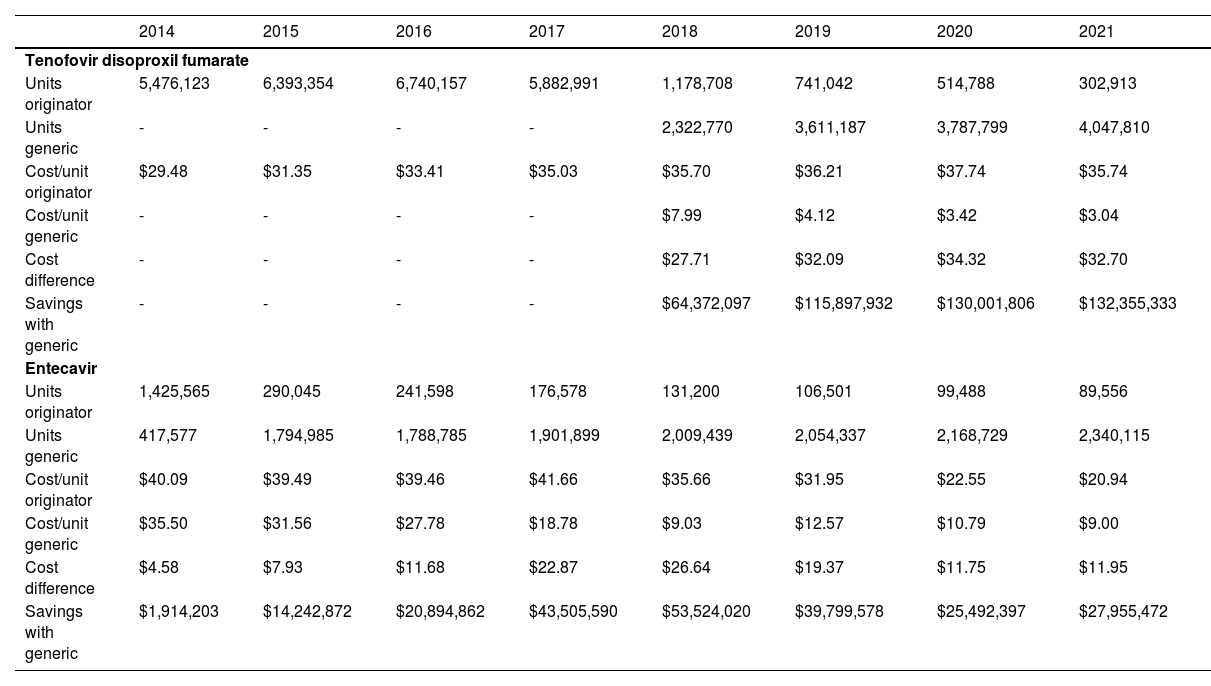
Specific analysis of expenditures between 2014-2021 showed that a total of $669 million USD was saved with the use of generics, with $443 million of savings specifically from the use of generic TDF (TDF reflects 83.4% of the total spending) (Table 2).
Estimated reduction in expenditure associated with generic entecavir and tenofovir disoproxil fumarate
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | |
|---|---|---|---|---|---|---|---|---|
| Tenofovir disoproxil fumarate | ||||||||
| Units originator | 5,476,123 | 6,393,354 | 6,740,157 | 5,882,991 | 1,178,708 | 741,042 | 514,788 | 302,913 |
| Units generic | - | - | - | - | 2,322,770 | 3,611,187 | 3,787,799 | 4,047,810 |
| Cost/unit originator | $29.48 | $31.35 | $33.41 | $35.03 | $35.70 | $36.21 | $37.74 | $35.74 |
| Cost/unit generic | - | - | - | - | $7.99 | $4.12 | $3.42 | $3.04 |
| Cost difference | - | - | - | - | $27.71 | $32.09 | $34.32 | $32.70 |
| Savings with generic | - | - | - | - | $64,372,097 | $115,897,932 | $130,001,806 | $132,355,333 |
| Entecavir | ||||||||
| Units originator | 1,425,565 | 290,045 | 241,598 | 176,578 | 131,200 | 106,501 | 99,488 | 89,556 |
| Units generic | 417,577 | 1,794,985 | 1,788,785 | 1,901,899 | 2,009,439 | 2,054,337 | 2,168,729 | 2,340,115 |
| Cost/unit originator | $40.09 | $39.49 | $39.46 | $41.66 | $35.66 | $31.95 | $22.55 | $20.94 |
| Cost/unit generic | $35.50 | $31.56 | $27.78 | $18.78 | $9.03 | $12.57 | $10.79 | $9.00 |
| Cost difference | $4.58 | $7.93 | $11.68 | $22.87 | $26.64 | $19.37 | $11.75 | $11.95 |
| Savings with generic | $1,914,203 | $14,242,872 | $20,894,862 | $43,505,590 | $53,524,020 | $39,799,578 | $25,492,397 | $27,955,472 |
Overall, spending on nucleoside/nucleotide analogues (NA) in Medicaid has decreased over the last decade despite stable claim numbers related to the increased use of generic NA therapy. The peak expense in 2016 relates both to the number of units prescribed that year and the high proportion of originators compared to generic units. Our study covers the COVID-19 period; the COVID-19 pandemic impacted healthcare delivery [7] and potentially may have led to identifying fewer individuals needing NA therapy due to decreased rates of presentation to medical attention. However, we do not feel that the pandemic played a major role in spending, given that overall spending for Medicaid increased in 2020 and 2021 [5].
Our study is limited by the fact that the indication and dosing for the medications are not reported. However, the only FDA approved indications for the use of lamivudine-HBV, adefovir, entecavir and tenofovir alafenamide (TAF) monotherapy are for the treatment of chronic HBV which have not changed since their introduction, although we acknowledge that they may be used for prophylaxis of HBV reactivation off label. As well, although TDF can be used to treat human immunodeficiency virus (HIV) infection, TDF monotherapy is not recommended for treatment or for HIV pre-exposure prophylaxis, so this current study data likely reflects HBV based NA treatment. This data is also limited by lack of publicly available information (due to proprietary / confidential industry negotiations) on the amount paid through the US Department of Health and Human Services Medicaid program for these drugs. Last, this dataset does not allow for assessing the proportion of Medicaid patients with HBV on treatment due to the denominator of patients with HBV and the number of patients receiving NA are not available. Previous estimates using data from 2007-2017 suggested a prevalence of 15.6 per 10,000 patients enrolled in Medicaid [8], which would equate to 135,000 patients with HBV on Medicaid in 2021, although with increased immigration [9], this likely is an underestimate of this population.
A recent analysis of HBV drug spending in Medicare Part D [1] showed similar trends in utilization in this older population, although tenofovir-based therapy made up 66% of the spending likely related to increased use of TAF in this series, as well, there was less use of lamivudine and adefovir in the Medicaid population as compared to Medicare [6]. Interestingly, the number of Medicare beneficiaries is increasing, unlike the current analysis. This difference may be related to the different populations enrolled in each program and a higher prevalence of HBV in older populations [10].
5ConclusionsIn conclusion, usage of NA for HBV treatment has a significant cost to Medicaid, but the economic burden of antiviral therapy for hepatitis B is decreasing overall. There is currently no cure for chronic hepatitis B, and affected individuals often require prolonged if not life-long, therapy. Moreover, some experts are advocating simplifying and expanding the threshold to start treatment for some individuals living with chronic hepatitis B, especially in older patients with detectable viral levels [11,12]. An increased understanding of the utilization of NA, as well as the impact of generic drug treatment to reduce morbidity and mortality, will improve understanding of evolving hepatitis B epidemiology nationwide and help in planning sustainable healthcare funding.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsSEC took overall responsibility for the study design, data analysis, and writing of the first draft, as well as for the conduct and coordination of the study. MB contributed to data analysis and participated in manuscript review and editing. Similarly, CSC also contributed to data analysis and participated in manuscript review and editing.
Data is publicly available.