Introduction. Hypersplenism in cirrhosis is not infrequent and may compromise with quality of life and therapy. Splenectomy is a therapeutic option, but information on results of splenectomy is scarce.
Material and methods. Consecutive patients with cirrhosis who underwent splenectomy between 2001-2010 were included in the study. Safety, efficacy of splenectomy and subsequent influence on therapy were evaluated.
Results. Thirty three patients (mean age 30.9 ± 11.6 years, 19 men, viral 48.5%, autoimmune 15.1%, cryptogenic 36.4%) underwent splenectomy. Twenty were Child's A, 13 Child's B. Twenty patients had > 6 months follow up. Common indications were inability to treat with interferon, transfusion-dependent anemia, recurrent mucosal bleeds, and large spleen compromising quality of life. Median hospital stay was 7 (4-24) days. There was no splenectomy related mortality. Twenty three (70%) patients had post-operative complications, most commonly infections. Two patients required percutaneous drainage of post-operative collections, and 1 needed re-exploration for intra-abdominal bleed. Subsequent to splenectomy platelet count (44,000 to 151,000/mm3, p < 0.01) and TLC (2,500 to 13,400/mm3, p < 0.01) had sustained increase in all patients except one. Five HCV cirrhotics completed interferon and ribavirin therapy, 4 achieved sustained viral response. The quality of life improved and there was no recurrence of infections, mucosal bleed or anemia requiring transfusions in any patient. In patients on long term follow up (median duration 27 months), the median Child's score improved from 6 at baseline to 5 at follow up (p < 0.05).
Conclusions. Splenectomy was safe and effective in patients with cirrhosis, and improved therapeutic options as well as Child's score.
Hypersplenism associated cytopenias in patients with cirrhosis are not infrequent and compromise with quality of life as well as limit therapeutic interventions. The frequency of cytopenias in cirrhosis of liver has been reported to vary between 11 to 64%.1
Hypersplenism induced thrombocytopenia may be associated with mucosal bleed. It may limit endotherapy for primary and secondary prophylaxis of variceal bleed, liver biopsy, and other surgical interventions. Anemia may result in transfusion dependence and poor outcome secondary to variecal bleed. Leucopenia may enhance infection risk in cirrhotics.2
Therapy with interferon and ribavirin is recommended in patients with compensated hepatitis C virus (HCV) related cirrhosis.3 A sustained virological response in such patients can retard the progression of disease and reduce the incidence of hepatocellular carcinoma.4,5 However, the cytopenia associated with HCV related Child's A cirrhosis may be a limitation for recommended treatment because of cytopenic side effects of interferon.6
Therapeutic options to alleviate hypersplenism in cirrhosis are limited. Growth factors available for managing anemia and thrombocytopenia include erythropoietin7 and Granulocyte colony stimulating factor (G-CSF)8 respectively. However, for thrombocytopenia even though eltrombopag has been found to be effective,9 it is not yet commercially available. Partial splenic artery embolization has been reported in case cohorts as a treatment for hypersplenism, but is associated with complications like intraabdominal abscess, splenic vein thrombosis, pleural effusion, and splenic rupture.10 It is also associated with recurrence of thrombocytopenia in these patients.11 Further, it needs skilled interventional radiologist and infrastructure. The said interventions are expensive and have limited access in resource poor set up.
Splenectomy is a conventional surgical procedure and may be widely available. However results of splenectomy in cirrhotics with hypersplenism are limited and their long term effects on the natural course of disease have not been described. Further, splenectomy may improve therapeutic options particularly in patients with HCV Cirrhosis, but the information on it is limited. Therefore, the present study evaluated the risk, benefits of splenectomy among patients with cirrhosis having hypersplenism and also evaluated the long term effect of splenectomy on the natural course of such patients. The study further evaluated whether splenectomy improves the therapeutic option, especially with regard to the treatment of hepatitis C, leading to overt clinical benefit.
Material and MethodsPatientsDemographic, clinical, radiological, laboratory, and histopathological data for all patients, with pre-operative diagnosis of cirrhosis with hypersplenism, who underwent splenectomy between 2001 to 2010, were retrieved from the medical and histopathological records at All India Institute of Medical Sciences, New Delhi, India. Cirrhosis was defined by conventional clinical, radiological, endoscopic or histology characteristics and their liver function status was graded using Child Turcotte Pugh's Score.12 We specifically looked at the aetiologies of cirrhosis, indications of splenectomy, post-operative complications, efficacy of splenectomy in improving cytopenia, and the influence of splenectomy on the natural course of disease as well as to whether it allowed specific therapeutic interventions using interferon and ribavirin in hepatitis C associated compensated cirrhosis. The etiologies of cirrhosis were ascertained using accepted criteria.13-16
Pancytopenia was defined as anemia (hemoglobin < 13.5 g/dL-male; 12 < g/dL-female); leucopenia (total leukocyte count < 4,000/mm3) and thrombocytopenia (platelet count < 150,000/mm3).
Peri-operative managementAn attempt was made to optimize the general condition of patient before surgery. Ascites was managed with low salt diet (< 2 g per day), diuretics, intravenous infusion of (20%) human albumin and paracentesis if required. Fresh frozen plasma was administered preoperatively to patients with prolonged prothrombin time. Preoperatively, none of our patients received vaccination against Pneumococcus, Meningococcus, or Hemophilus influenzae. This was based on our previous experience with splenectomy for portal hypertension in patients with Extra Hepatic Portal Venous Obstruction (EHPVO)17 and Non Cirrhotic Portal Fibrosis (NCPF)18 in whom overwhelming post splenectomy sepsis (OPSI) was not documented over long period of follow up.
Splenectomy and liver biopsy were performed under general anaesthesia in supine position through a left subcostal incision. A total splenectomy (including removal of splenunculus if present) was done by open surgical technique. Devascularisation or left gastric vein ligation was not done routinely. Devascularisation was done in patients with high grade varices. Prophylactic antibiotics (amoxicillin/ clavulininc acid and amikacin) were administered at induction and continued postoperatively if indicated. After division of gastrocolic ligament, splenic artery was doubly ligated in continuity at the upper border of pancreas before splenic mobilization. Platelet and blood transfusions were administered at this stage if required. Splenectomy was done by division of its vascular attachments. Drains were placed selectively. Needle liver biopsy was taken in all patients and bleeding from liver surface controlled with argon coagulation or haemostatic sutures. Postoperatively, patients were managed in a high dependency unit. Oral feeds were started on postoperative day 1 and increased as tolerated. Ascites was managed with albumin infusions and diuretics. Drains were removed once drainage fluid was not blood stained and < 500 mL/day.
The post-operative complications were classified according to Clavien-Dindo classification.19
Management at follow upEach patient was followed up at our out patients clinic at 4 weeks interval for 3 months and subsequently at 3 months interval. During every follow up, each patient had thorough clinical examination, complete blood count, liver function test, prothrombin time and Child's Turcotte Pugh (CTP) score assessment. Patient data was maintained in a liver file. Patients received standard management for their respective disease aetiologies, and complications like variceal bleed, infection, encephalopathy, and ascites.
Statistical analysisAll continuous data values were expressed as mean ± standard deviation and categorical variables were expressed as percentage. Statistical comparisons between pre-operative, post-operative and follow up hemoglobin, platelet count, and total leukocyte count were made using paired t-test. The pre-operative and follow up child's scores were compared using non parametric test (Wilcoxon signed ranks test). A p value less than 0.05 was considered statistically significant.
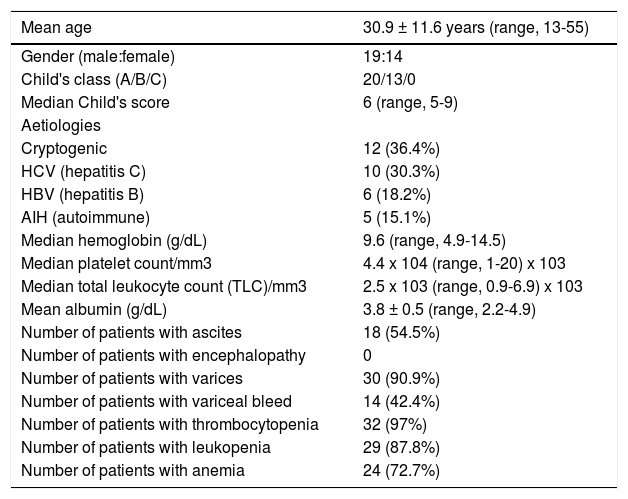
ResultsBaseline clinical and demographic characteristicsThirty three patients of cirrhosis underwent splenectomy between 2001 and 2010. Mean age of the patients was 30.9 ± 11.6 years. Nineteen (57.5 %) patients were males. Twenty (60.6 %) patients had Child's A status and 13 (39.4 %) had Child's B status. The aetiologies of cirrhosis were hepatitis C (HCV) in 10 (30.3%), hepatitis B (HBV) in 6 (18.2%), autoimmune liver disease (AIH) in 5 (15.1%) and cryptogenic in 12 (36.4%) patients (Table 1). The median Child's score at baseline was 6. Follow up data for > 6 months was available for 20 (60.6 %) patients. Maximum patients had thrombocytopenia (97 %) followed by leukopenia (87.8 %) and anemia (72.7 %). Majority of patients (72.7 %) had pancytopenia.
Baseline demographic and clinical characteristics of patients undergoing splenectomy (n = 33).
| Mean age | 30.9 ± 11.6 years (range, 13-55) |
|---|---|
| Gender (male:female) | 19:14 |
| Child's class (A/B/C) | 20/13/0 |
| Median Child's score | 6 (range, 5-9) |
| Aetiologies | |
| Cryptogenic | 12 (36.4%) |
| HCV (hepatitis C) | 10 (30.3%) |
| HBV (hepatitis B) | 6 (18.2%) |
| AIH (autoimmune) | 5 (15.1%) |
| Median hemoglobin (g/dL) | 9.6 (range, 4.9-14.5) |
| Median platelet count/mm3 | 4.4 x 104 (range, 1-20) x 103 |
| Median total leukocyte count (TLC)/mm3 | 2.5 x 103 (range, 0.9-6.9) x 103 |
| Mean albumin (g/dL) | 3.8 ± 0.5 (range, 2.2-4.9) |
| Number of patients with ascites | 18 (54.5%) |
| Number of patients with encephalopathy | 0 |
| Number of patients with varices | 30 (90.9%) |
| Number of patients with variceal bleed | 14 (42.4%) |
| Number of patients with thrombocytopenia | 32 (97%) |
| Number of patients with leukopenia | 29 (87.8%) |
| Number of patients with anemia | 24 (72.7%) |
The most common indication for splenectomy was inability to treat with interferon and ribavirin in 10 patients with HCV related cirrhosis. Other indications were severe thrombocytopenia causing recurrent mucosal bleed (epistaxis/gum bleed) in 5 patients; recurrent overt variceal bleed requiring transfusions in 4 patients; transfusion dependent anemia (without any GI bleed) in 2 patients. In 10 patients the indication was large spleen causing upper abdominal discomfort, interference with nutrition and compromised ambulation. One patient had recurrent infections possibly due to leukopenia, and one patient required splenectomy because of splenic laceration and hematoma.
Peri operative and post operative eventsMean operative time was 153.3 ± 46.5 (range, 65-260) min. The mean intra operative blood loss was 600 ± 476 (100-2,000) mL. Mean and median post operative hospital stay was 9.2 ± 5.8 days and 7 (4-24) days respectively. Twelve out of 33 (36.3 %) patients required blood transfusion in the intraoperative period.
Post operative course was uneventful in 10 (30.3 %) patients. Ten (30.3 %) patients developed fever in the post operative period. Of these, 2 patients had intra abdominal collections requiring per-cutaneous drainage, 4 patients had pneumonia, one patient had wound infection. In 3 patients exact cause of fever could not be determined, but they responded to empirical antibiotics.
Nine patients had ascites, 4 patients had prolonged drainage through the abdominal drain and high drain output, which resolved with diuretics. Two patients had transient hepatic encephalopathy. One patient had bleed from incision site, which required re-exploration that revealed bleed from liver biopsy site. One patient each had urinary catheter induced hematuria, vocal cord injury causing hoarseness of voice, and right sided pleural effusion. Seven patients had more than one complication. Thus, 5 patients had grade I, 15 patients had grade II, 2 (6%) patients had grade Ilia and 1(3%) patient had grade IIIb complication according to Clavien-Dindo classification. The median hospital stay was 5 (range, 4-8) days in patients without no complication or grade I complication, 10 (range, 5-21) days in patients with grade II complication, and 24 (range, 5-24) days in patients with grade III complication. There were no in-hospital deaths. Each patient with morbidity due to splenectomy could be managed effectively and was discharged from hospital in an ambulatory state.
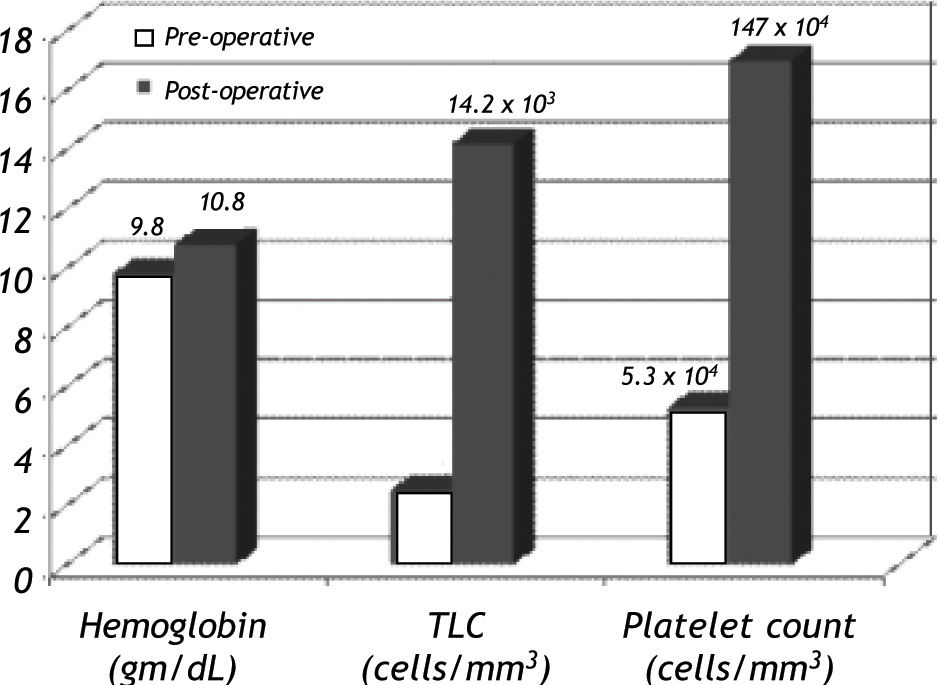
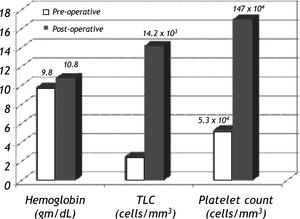
Response to splenectomyThere was significant improvement in hemoglobin, total leukocyte count (TLC) and platelet count after 48 h of splenectomy (p < 0.05) (Figure 1). The median hemoglobin, TLC and platelet count increased from 9.6 gm/dL (range, 4-14.5), 2.5 x 103/mm3 (range, 0.9-6.9 x 103), and 4.4 x 104/ mm3 (range, 1-20 x 104) respectively at baseline to 10.8 gm/dL (range, 5.7-14.6), 13.4 x 103/mm3 (range, 4.8-38 x 103), and 15.1 x 104/mm3 (range, 7.9-33.5 x 104) respectively in post operative period.
Long term follow upLong term follow up > 6 months was available in 20 patients and these patients were included in analysis for evaluating long term results of splenectomy. Median follow up was 27 (8-105) months.
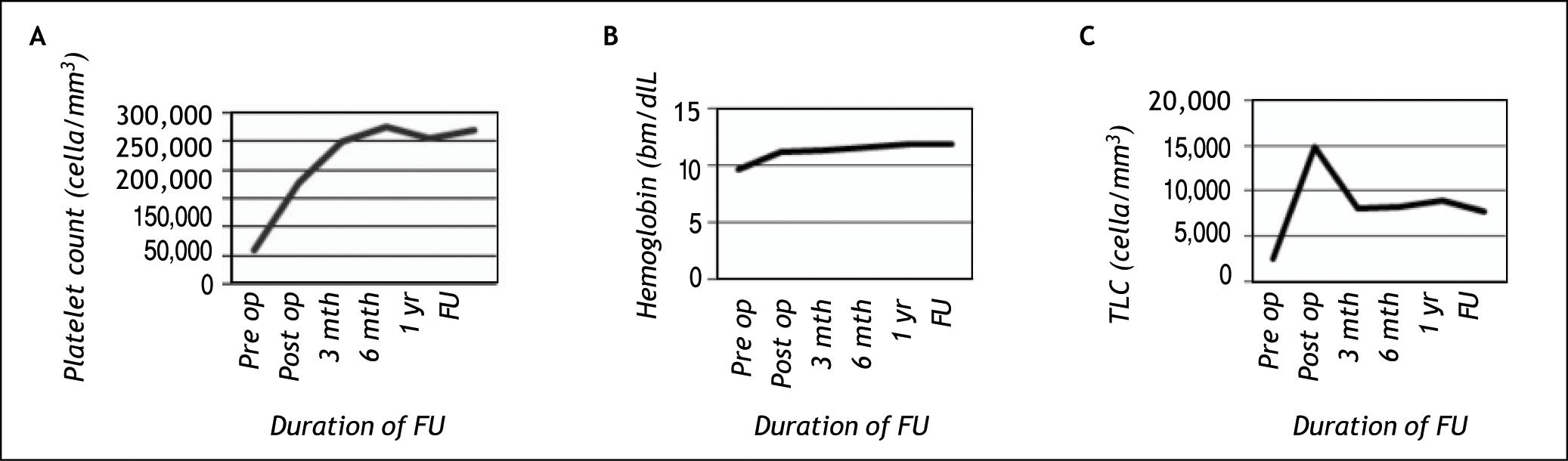
Hematological responseThere was significant improvement in all haematological parameters which was sustained till the end of follow up in all patients except one (p < 0.05) (Figure 2). The median hemoglobin, total leukocyte count (TLC) and platelet count increased from 9.5 gm/dL (range, 4.9-12.9), 2.5 x 103/ mm3 (range, 1-6.9 x 103) and 5.8 x 104/mm3 (range, 1.7-20 x 104) respectively at baseline to 10.9 g/dL (range, 7.8-14.6), 13.4 x 103/mm3 (range, 4.8-38 x 103), and 15.8 x 104/mm3 (range, 8.6-33.5 x 104), respectively in post operative period, and was 12.2 g/dL (8.9-15.4), 7.2 x 103/mm3 (range, 4.4-13.1 x 103), and 27.5 x 104/mm3 (range, 4-56.8 x 104) respectively at follow up. The patient in whom the cytopenia did not improve had hypocellular bone marrow on follow up.
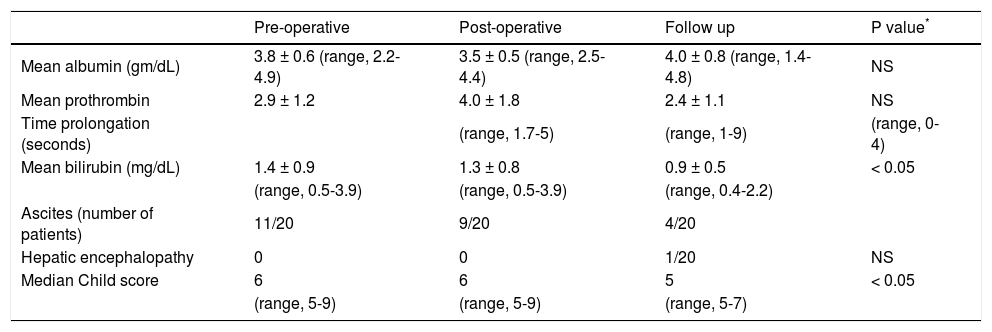
Change in child’s score after splenectomyThe child’s score deteriorated post operatively, but at follow up the child score improved in 11 (55 %) patients (Table 2). There was significant improvement in median Child's score at follow up (6 to 5, p < 0.05).
Change in Child's score after splenectomy in patients on long term follow up (n = 20).
| Pre-operative | Post-operative | Follow up | P value* | |
|---|---|---|---|---|
| Mean albumin (gm/dL) | 3.8 ± 0.6 (range, 2.2-4.9) | 3.5 ± 0.5 (range, 2.5-4.4) | 4.0 ± 0.8 (range, 1.4-4.8) | NS |
| Mean prothrombin | 2.9 ± 1.2 | 4.0 ± 1.8 | 2.4 ± 1.1 | NS |
| Time prolongation (seconds) | (range, 1.7-5) | (range, 1-9) | (range, 0-4) | |
| Mean bilirubin (mg/dL) | 1.4 ± 0.9 | 1.3 ± 0.8 | 0.9 ± 0.5 | < 0.05 |
| (range, 0.5-3.9) | (range, 0.5-3.9) | (range, 0.4-2.2) | ||
| Ascites (number of patients) | 11/20 | 9/20 | 4/20 | |
| Hepatic encephalopathy | 0 | 0 | 1/20 | NS |
| Median Child score | 6 | 6 | 5 | < 0.05 |
| (range, 5-9) | (range, 5-9) | (range, 5-7) |
The quality of life improved after splenectomy and there was no recurrence in infection, anemia requiring recurrent blood transfusions or mucosal bleed in any patient who underwent splenectomy for respective indications.
Twelve patients (60%) had an uneventful follow up. None of the patients had overwhelming post splenectomy sepsis (OPSI) during follow up. Two patients developed hepatocellular carcinoma (HCC) on follow up, which was diagnosed as per European Association for Study of Liver Disease (EASL)20 criteria, and were appropriately treated as per Barcelona Clinic Liver Cancer (BCLC)21 staging and treatment allocation. One of them died in a road traffic accident, 62 months after splenectomy. The other patient is still under follow up, and has had no recurrence of HCC. Four patients decompensated with ascites which could be controlled on conservative medical management. Of these, one patient developed an episode of spontaneous bacterial peritonitis, one patient developed portal vein thrombosis, and other had an episode of encephalopathy, which could be managed effectively. One patient had recurrence of pancytopenia, and on follow up his bone marrow revealed hypocellular marrow. He is currently under follow up and is on appropriate treatment under hematologist. There have been 2 deaths. Both are related to causes unrelated to liver disease. One patient died in road traffic accident as mentioned earlier. Other patient died due to homicidal poisoning.
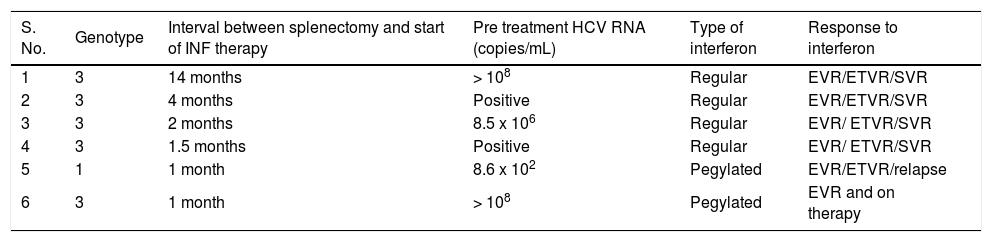
Interferon treatment after splenectomySubsequent to splenectomy 6 out of 10 patients with HCV cirrhosis could be treated with interferon and ribavirin without any need for interruption of therapy or dose modification (Table 3). Two patients received pegylated interferon and 4 patients received regular interferon as they could not afford pegylated interferon. Five patients achieved End of Treatment Virological Response (ETVR),14 and 4 had sustained virological response (SVR).14 One patient had viral relapse after achieving ETVR. She had HCV genotype 1 infection. Sixth patient is on therapy and has achieved early virological response (EVR).14 Another 2 patients who had improvement in their cytopenias are planned for interferon therapy. One patient was lost to follow up. In one patient there was a relapse of pancytopenia, and on further evaluation he was detected to have hypocellular marrow. He is currently under follow up and is on appropriate treatment.
Results of interferon and ribavirin treatment in patients with HCV-cirrhosis after splenectomy.
| S. No. | Genotype | Interval between splenectomy and start of INF therapy | Pre treatment HCV RNA (copies/mL) | Type of interferon | Response to interferon |
|---|---|---|---|---|---|
| 1 | 3 | 14 months | > 108 | Regular | EVR/ETVR/SVR |
| 2 | 3 | 4 months | Positive | Regular | EVR/ETVR/SVR |
| 3 | 3 | 2 months | 8.5 x 106 | Regular | EVR/ ETVR/SVR |
| 4 | 3 | 1.5 months | Positive | Regular | EVR/ ETVR/SVR |
| 5 | 1 | 1 month | 8.6 x 102 | Pegylated | EVR/ETVR/relapse |
| 6 | 3 | 1 month | > 108 | Pegylated | EVR and on therapy |
EVR: early virological response. ETVR: end of treatment virological response. SVR: sustained virological response. HCV: hepatitis C virus. INF: interferon.
Hypersplenism in cirrhosis is a clinical problem influencing the natural course of disease and therapeutic decisions. It can hinder the antiviral therapy in compensated HCV-cirrhosis. Quality of life in such patients is compromised due to anemia requiring multiple transfusions, thrombocytopenia leading to recurrent mucosal bleeds, and large spleen causing upper abdominal discomfort and restricted ambulation. Patients with leukopenia may be at increased risk of infection.
The present study documented that splenectomy in patients with cirrhosis having good liver function (Median Child’s Score 6) (Table 1), associated with hypersplenism, is safe and could correct cytopenias in all patients at 48 h of splenectomy. The improvement of cytopenias was sustained over a median follow up of 27 (8-105) months in all patients except one. The results of splenectomy in improving cytopenias have been described earlier,22-25 and recent series have documented its superiority over partial splenic artery embolization.26,27 However, laparoscopic splenectomy recently has been documented to be more frequently practiced,25,26 although, studies have shown comparable efficacy with either approach.23
The present study documented two distinct aspects of splenectomy which are scarcely documented in the literature. The present study documented that over a median follow up period of 27 months the median child's score improved from 6 to 5 (Table 2). Such an event could have been due to successful post splenectomy treatment using interferon and ribavirin in HCV-cirrhotics, who were previously not eligible for such therapy due to cytopenias. Ten patients (30 % of patients included in the present study) with HCV-cirrhosis, subsequent to splenectomy had significant improvement in platelet count and TLC at 48 h of surgery, and 5 of them had appropriate therapy, of whom 4 achieved SVR. One patient under treatment also had EVR and the remaining patient with genotype 1 infection relapsed. In all these patients improvement in liver functions was documented which may have improved the Child's score.
Although the morbidity rate in our series was 70%, it rarely resulted in prolonged hospital stay, and all patients were discharged after a median hospital stay of 7 days. Only three patients (9%) required any invasive intervention for management of complications.
Till date post splenectomy treatment of HCV infection has been described in few reports which have emanated from regions with genotype 1 HCV infection. In India genotype 3 is the prevalent HCV genotype.28 The results of treatment of genotype 3 HCV infection have already been documented to be better than that in genotype 1 infection.29 Therefore, in patients with HCV cirrhosis with cytopenia due to hypersplenism, which is a deterrent for specific treatment using Interferon and ribavirin, splenectomy may be an effective option particularly in patients with genotype 3 associated HCV cirrhosis. Indeed the present study documented that in almost all patients with HCV cirrhosis the cytopenias were corrected and 9 (90%) became eligible as well as suitable for such treatment. Further, among those who received treatment all had early viral response, 83% (5/6) had ETVR, and 80% (4/5) had SVR (Table 3). Even though the numbers of patients are small, the results are encouraging.
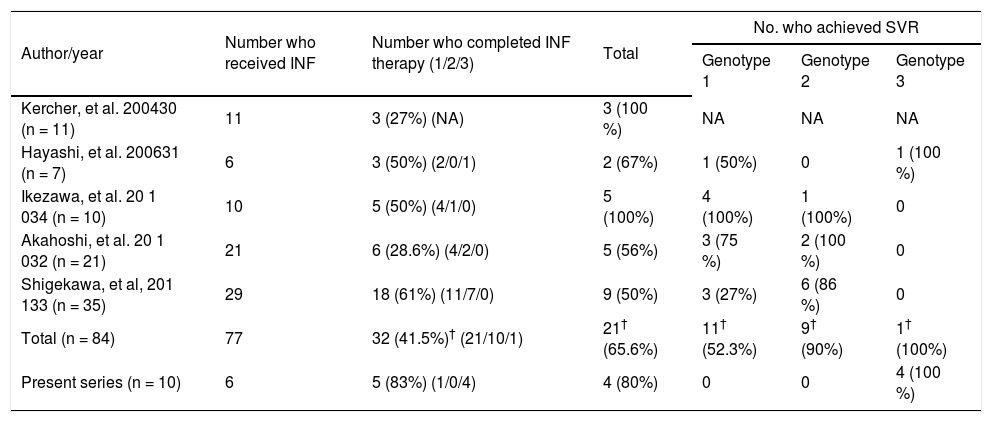
There are 5 reports which have documented improvement in haematological parameters subsequent to splenectomy in total of 84 patients with HCV related cirrhosis30-34 (Table 4). Out of 84 such patients, interferon and ribavirin could be instituted in 77 patients and completed in 35 (49%) patients. However, information on HCV genotype was available in 32 of 35 patients. Twenty one (65.6%) and 11 (34.4%) patients had genotype 1 and genotype 2/3 infection (10 genotype 2, 1 genotype 3) respectively. SVR was documented in 65.6% (21/32) of these patients. Expectedly, SVR among genotype 1 infected patients was lower than that in patients infected with genotype 2/3 HCV (52.3% vs. 91%) (Table 4). However all the reports included only one patient with genotype 3 HCV infection and the present study documented the beneficial effect of spenectomy in making such patients eligible for specific therapy with benefit.
Results of interferon and ribavirin treatment in HCV-cirrhosis after splenectomy (previously reported in literature).
| Author/year | Number who received INF | Number who completed INF therapy (1/2/3) | Total | No. who achieved SVR | ||
|---|---|---|---|---|---|---|
| Genotype 1 | Genotype 2 | Genotype 3 | ||||
| Kercher, et al. 200430 (n = 11) | 11 | 3 (27%) (NA) | 3 (100 %) | NA | NA | NA |
| Hayashi, et al. 200631 (n = 7) | 6 | 3 (50%) (2/0/1) | 2 (67%) | 1 (50%) | 0 | 1 (100 %) |
| Ikezawa, et al. 20 1 034 (n = 10) | 10 | 5 (50%) (4/1/0) | 5 (100%) | 4 (100%) | 1 (100%) | 0 |
| Akahoshi, et al. 20 1 032 (n = 21) | 21 | 6 (28.6%) (4/2/0) | 5 (56%) | 3 (75 %) | 2 (100 %) | 0 |
| Shigekawa, et al, 201 133 (n = 35) | 29 | 18 (61%) (11/7/0) | 9 (50%) | 3 (27%) | 6 (86 %) | 0 |
| Total (n = 84) | 77 | 32 (41.5%)† (21/10/1) | 21† (65.6%) | 11† (52.3%) | 9† (90%) | 1† (100%) |
| Present series (n = 10) | 6 | 5 (83%) (1/0/4) | 4 (80%) | 0 | 0 | 4 (100 %) |
n: total number of patients in each series. (1/2/3): Proportion of patients with genotype 1, 2 and 3 respectively. †Excludes patients from series by Kercher, et al.27as HCV genotype was not available. SVR: sustained virological response. HCV: hepatitis C virus. NA: not available. INF: interferon.
These results would indicate that splenectomy is a safe option to make patients with cirrhosis and hypersplenism eligible for specific antiviral treatment against hepatitis C virus infection, particularly in patients infected with genotype 3 virus.
The second important observation in the present study was absence of documentation of overwhelming post splenectomy sepsis in any of the patients during a median follow up of 27 months, despite the fact that, none of the patients received pneumococcus, meningococcus or Hemophilus influenzae vaccination prior to surgery, suggesting long term safety of the procedure. Vaccination against above organisms, prior to splenectomy, is usually recommended to prevent the occurrence of overwhelming post splenectomy sepsis (OPSI).35 However, the previous results of splenectomy in patients of EHPVO and NCPF at our centre reveal no incidence of OPSI after splenectomy. There was no incidence of OPSI in 45 patients of NCPF who underwent splenectomy and were followed up for median duration of 24 months.18 Similarily OPSI was not documented in 117 patients of EHPVO who were followed up from 2 to 10 years after splenectomy17. Therefore, looking at our previous results we did not vaccinate any patient.
Further, 60 % patients included in the present report had improvement in their Child's score (Table 2) without unwarranted events.
In conclusion, the present study documented that splenectomy in compensated cirrhotics with hypersplenism was safe, corrected cytopenias, made HCV cirrhotics eligible for specific antiviral treatment with SVR rate of 80 % in those who received treatment, and improved Child's score at long term follow up.
Conflict of InterestNone to declare