Introduction. Cholestasis leads to liver cell death, fibrosis, cirrhosis, and eventually liver failure. Bile duct ligated rats constitute an interesting model to study the mechanism of cholestasis, and its action on several organs and tissues, including the brain.
Aim. To analyze brain bile acids individually in ligated rats to evaluate if its profile is altered towards a more toxic condition in cholestasis.
Material and methods. Male Wistar rats were used and separated in two groups: bile duct ligated rats and sham operated rats (n = 5 in each group). Bile acid profile was assessed in brain homogenates. Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase determinations, bilirubin and ammonia plasma concentration were also measured in both groups.
Results. Although the total amount of bile acids in control animal brains showed a higher concentration than in bile duct ligated rats, the bile acid profile in this group was found more toxic composition than in controls. Lithocholic acid was present in brain in higher concentration (87.4 % of total brain bile acids) in ligated rats and absent in controls. Alkaline phosphatase, bilirubin and ammonia were significantly higher in bile duct ligated rats than in control group.
Conclusion. It was found a toxic brain bile acid profile during hepatic cholestasis which could be one of the explanations of hepatic encephalopathy observed in cholestatic diseases.
Cholestasis is a liver pathology produced when an interruption of bile flow in small ducts or extra hepatic bile ducts, takes place and then, bile contents cannot reach the intestine returning backward to plasma. There are several major complications in hepatic chronic disease: portal hypertension found in abdominal splachnic circulation, ascites, endocrine manifestations and hepatic encephalopathy (HE) due to the presence of toxic substances such as ammonia, proinflammatory cytokines and free radical productions.1 HE constitutes an intriguing complication in severe acute and chronic liver disease. A wide range of psychoneurological symptoms are usually associated to brain edema and intracranial hypertension as well as changes of the function of brain cells.1,2 In cholestasis, bilirubin, bile acids (BAs) and other toxins of hepatic failure were found in systemic circulation reaching near and distant tissues. Diffusion of some of these liver metabolites reach central nervous system (CNS) increasing the blood brain barrier (BBB) permeability3,4 and, thus, permitting the passage of toxic substances from the blood to the brain. As a result, BAs could penetrate brain promoting their toxic effects.
BAs are amphipathic molecules synthesized from cholesterol metabolism, formed in the hepatic parenchyma with very reactive structure acting on cellular membranes.
Previous studies have demonstrated the existence of unconjugated cholic acid (CA), deoxicholic acid (DCA) and chenodeoxicholic acid (CDCA) in rat brain.5 These authors suggest that BAs with a previously unrecongnized action, may play a significant role in the central nervous system.
BAs toxicity is generally associated with their amphiphilic proprieties. The balance between hydro-phobic and hydrophilic characters differs depending on the bile acid species composition6,7 being lithocholic acid (LCA) the most hydrophobic bile acid and, therefore, the most toxic one possessing damaging effects on vital tissues like the CNS. So early in 1969 Naqvi, et al.8 demonstrated the demyelinating and paralytogenic action when LCA was injected intracerebrally. However, to our knowledge the complete brain bile acid profile was not studied in a cholestatic condition.
To expand these previous findings, the major goal of this research was to examine the brain bile acid profiles when the major bile excretory route is completely blocked by long-term bile-duct obstruction.
Material and MethodsMale Wistar rats were utilized for this experiment, with an average weight of 230-240 g. The animals were treated in accordance with guideline of a Committee of animal care of the Faculty of Pharmacy and Biochemistry of Buenos Aires, according to Helsinki rules.
Animals were placed in individual cages during recovery from operations and had free access to food and water. A 12 h of light-dark cycle (8 am-8 pm) was applied.
Rats were divided in two groups of 5 rats each: bile duct ligated rats (BDL group) and, sham operated rats (control group). BDL rats, were operated under light ether anesthesia, and a double surgical ligation, were placed (2 silk knots proximal to bifurcation were tied on common duct), and sectioned between both knots. Sham operated rats, were anesthetized with ether, laparotomized and bile ducts were recognized but untied or uncut.
Twenty one days after operation the rats of both groups, under light, ether anesthesia were submited to a splanchnic pulp cannulation to measure the portal pressure.
Portal pressure measurementPortal pressure was measured through a needle placed in the splenic pulp and maintained in place by cyanoacrylate gel. The needle was connected to a polyethylene catheter 50 filled with a heparinized saline solution (25 V/mL), and connected to a Statham Gould P231D pressure transducer (Statham, Hato Rey, Puerto Rico) coupled to a grass 79 D polygraph (grass instruments, Quincy MA, U.S.A).
After this procedure all rats were sacrificed by decapitation. A plasma sample and total brain were obtained for biochemical determinations.
Bile acid determinationsTotal and individual bile acids in serum and brain homogenates: cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA) and ursodeoxycholic acid (UDCA), in their free, glycine and taurine derivative forms were assessed by capillary electrophoresis. A detailed description of the analytical method performed in this study has been described by Tripodi, et al.9 Briefly, simultaneous determinations of 15 bile acids were performed using an off-line C18 solid phase extraction procedure for sample clean-up and concentration. This step was followed by the complete separation of the bile acids using cyclodextrine-modified micellar electrokinetic chromatography with UV detection and quantitation was accomplished in less than 12 min.
Biochemical determinationsThe activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase and bilirubin were determined in plasma. Biochemical parameters were determined using standardized and optimized commercial Roche kits. Plasma ammonia concentration was determined using commercial Randox kit.
Statistical analysisDescriptive statistics for continuous variables included median (quartiles 25-75), while for categorical variables, proportions or percentages were employed. For comparisons of continuous variables between the groups a nonparametrical test (Mann Whitney U test), was used.
ResultsBDL rats showed higher plasma activities of alkaline phosphatase than control group: 858.0 (697.0-952.0) vs. 351.5 (347.0-363.5) IU/L (p < 0.01), respectively. BDL rats also showed raised plasma levels of bilirubin than control groups: 0.945 (0.4201.980) vs. 0.275 (0.225-0.340) mg/dL, (p < 0.03) together with higher levels of plasma ammonia: 85.7 (70.7-100.7) vs. 25.1 (15.9-34.3) μM, respectively (p < 0.01).
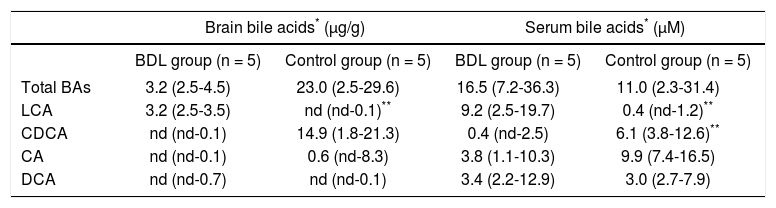
Brain bile acidsIn brain of the control group it was observed a prevalence of primary the bile acids CA and CDCA in their free and in their conjugated forms with glycine and taurine.
In brain of BDL rats it was found LCA in a higher proportion (free and its tauroderivative form, TLCA) and DCA (free and its tauroderivative form, TDCA). Free and conjugated LCA only was detected in brain of cholestatic rats, showing this bile acid to be 87.4% of the total bile acids contents.
Table 1 shows the quantitative profile of brain bile acids in control and BDL rats.
Brain and serum bile acid profiles in control and cholestatic rats.
| Brain bile acids* (μg/g) | Serum bile acids* (μM) | |||
|---|---|---|---|---|
| BDL group (n = 5) | Control group (n = 5) | BDL group (n = 5) | Control group (n = 5) | |
| Total BAs | 3.2 (2.5-4.5) | 23.0 (2.5-29.6) | 16.5 (7.2-36.3) | 11.0 (2.3-31.4) |
| LCA | 3.2 (2.5-3.5) | nd (nd-0.1)** | 9.2 (2.5-19.7) | 0.4 (nd-1.2)** |
| CDCA | nd (nd-0.1) | 14.9 (1.8-21.3) | 0.4 (nd-2.5) | 6.1 (3.8-12.6)** |
| CA | nd (nd-0.1) | 0.6 (nd-8.3) | 3.8 (1.1-10.3) | 9.9 (7.4-16.5) |
| DCA | nd (nd-0.7) | nd (nd-0.1) | 3.4 (2.2-12.9) | 3.0 (2.7-7.9) |
In BDL rats total BAs were found in lower concentrations than controls, however its profile showed to be more lipophilic. BDL rats showed a predominant presence of secondary over the primary bile acids in brain and the relationship between secondary and primary bile acids was 16.7.
In BDL rats the ratio between unconjugated/conjugated bile acids was 0.79 but in sham operated rats it was 0.23 showing an increase of free bile acids, which means a higher proportion of lipophilic bile acids in ligated rats.
Serum bile acidsTable 1 shows the quantitative profile of serum bile acids in control and BDL rats.
In serum, it was found the same profile as in brain. It was observed that the ligation produces a shift towards a lipophilic pattern showing an increment of LCA and DCA and a decrease in CA and CDCA levels.
Portal pressure resultsA significant increase in portal pressure was found in BDL rats: 12.1 ± 1.0 mmHg as compared to 7.6 ± 0.2 mmHg (P < 0.01) in sham operated rats.
DiscussionCommon bile duct ligation represents a well-defined experimental model of extrahepatic cholestasis. In our work, increased activities in plasma ALT and AST suggest, as it was expected, the presence of damage in liver parenchyma. Moreover, higher plasma bilirubin levels and elevated activities of ALP are biochemical signs of cholestasis. It was also observed a significative increase of plasmatic ammonia levels which is a major pathophysiological factor in encephalopathies associated with acute and chronic liver failure.
There are few contributions regarding to the levels of BAs and their profile in tissues under cholestatic condition. Otherwise it is very difficult to find an analytical system capable to measure, with high selectivity and sensitivity, the individual bile acid concentrations. Capillary electrophoresis is a high quality technique even more than HPLC-tandem mass spectrometry because of its simplicity and the possibility to determine simultaneously BAs univocally.
Therefore, the goal of the present study was to measure the bile acid profile in brain using capillary electrophoresis as analytical technique in an extrahepatic cholestasis model produced in long term ligated rats.
The first interesting finding in this investigation is that the total amount of BAs in brain tissue in sham operated animals appears to be higher than in BDL rats. In previous works we have demonstrated that total content of BAs is not enough a representative parameter because of its high interindividual variability and, independently of its levels, the profile of individual bile acid should be taken into account for a correct interpretation of the results.10,11
In the present work, analyzing the concentration of individual bile acids by capillary electrophoresis, we have found a high LCA concentration in brain of BDL rats, corresponding to the 87.4 % of the total brain BAs. Amounts of its conjugates with taurine were also in high concentrations. However, LCA was not detectable in brain from control rats. To our knowledge this is the first time that this toxic brain bile acid profile is found during hepatic cholestasis. This finding was possible due to the advantages of using capillary electrophoresis. Due to the high performance characteristics of this methodology is possible to quantify bile acids with high hydro-phobicity as LCA which is highly difficult by other methodologies.
In a previous work,11 we have also suggested that ammonia is not the only agent that induce HE, but several other substances such as glutamate, glutamine and oxidative stress could participate in the development of the encephalopathic syndrome in acute and chronic liver damage.
On the basis of our results we hypothesized that LCA could act as another possible toxic compound to the BBB and brain and that it may participate in the pathogenesis of HE. There are many evidences that support the hypothesis related to the toxicity of LCA.
LCA is a monohydroxylated secondary bile acid which is formed from the primary bile acid CDCA and it is one of the most hydrophobic naturally occurring Bas.12 In humans, systemic LCA concentrations are normally less than 1 μM, however, during cholestatic hepatobiliary disorders, LCA is raised as it was observed in the present work.
Moreover, it has been demonstrated by Ceryak, et al.,13 that extrahepatic LCA uptake was increased in many organs, including total brain, respect to sham (control) animals in 2-day bile duct ligated hamsters using 14C LCA. In our 21-day BDL rats model, brain LCA was increased in agreement with this work mentioned.
The same authors have demonstrated that BAs uptake increase as a function of decreasing polarity (decreasing hydroxylation), this is probably caused by differences in the transbilayer mobility of BAs which is inversely related to the number of their hydroxyl groups. These observations could explain the different rate of uptake of BAs with the predominance of LCA in brain showed in our study.
The elevation of hydrophobic BAs concentration in brain, that we have found, suggests their contribution in the mechanism of brain damage, leading to functional alteration, due to their physicochemical proprieties as it was previously mentioned by Krähenbühl, et al. in 1995.14
In accordance to this statement, bile acid-induced membrane damage has been reported to positively correlate with the degree of hydrophobicity and detergent effect of each particular bile acid.15
Hydrophobic BAs can enter cells by sodium-independent transporter as well as by passive diffusion, which is a function of their respective hydrophobicity.16,17 Through this latter mechanism, specially the most hydrophobic BAs like LCA, can pass through the cellular plasma membranes of tissues.
It is well appreciated that long-term exposure of cells to non-polar BAs is associated with cytotoxicity. The toxicity of BAs increase in cells which does not possess either a bile acid transporter or an efficient system for binding and/or detoxifying these compounds, as it occurs in brain.18
Moreover, it was demonstrated that mitochondrial damage is implicated in the toxicity of CDCA and LCA even under condition of a short time exposure.13 These authors, using the same BDL model as in our work, concluded that enhanced uptake of hydrophobic bile acids in tissues, including brain, could result in pathological effects for the targeted organs.
According to Krähenbühl, et al.19 lipophilic bile acids, such as LCA, reduce the activity of the electron transport chain in mitochondria. This reduction could lead to increase oxygen radical production, which by itself can be associated with a reduction in the activities of complexes I and III of the electron transport chain.
Experiments of same author also showed that a mitochondrial dysfunction is present in common bile duct ligated rats, suggesting the presence of an oxidative damage in these organelles, including in brain tissue.14
Weil, et al.,20 so early as in 1929 noted that taurocholic acid (TCA) produced a marked demyelination in vitro, and suspected that some other type of bile acid can produce this effect in vivo when they are synthesized in brain or introduced through brain circulation. Later, Nicholas, et al.21 confirmed this finding and Naqvi, et al. in 1969 observed the demyelinating effect of LCA.8
Our finding of a high concentration of LCA in brain of BDL rats suggests that in cholestasis this toxic substance is capable to cross the BBB, to enhance its damaging effects on CNS and to possibly aggravate the hepatic encephalopathy.
ConclusionsUsing a rat long-term cholestatic model we found that LCA is present in brain in high concentration corresponding to 87.4 % of total brain BAs. It was also found a high concentration of its conjugated forms with taurine (TLCA) in BDL rats against the absence of LCA in sham operated rats. Liposolubility and detergent properties of LCA can explain its passage through the BBB. These increments in brain make possible that both BAs may produce alterations in cells membranes and could participate in the pathogenesis of HE observed in cholestatic diseases.
AcknowledgementsThe authors wish to thank Dr. Jorge Muse for English assistance.
Abbreviations- •
CA: cholic acid.
- •
CDCA: chenodeoxycholic acid.
- •
DCA: deoxycholic acid.
- •
GCA: glycocholic acid.
- •
GCDCA: glycochenodeoxycholic acid.
- •
GDCA: glycodeoxycholic acid.
- •
GLCA: glycolithocholic acid.
- •
LCA: lithocholic acid.
- •
TCA: taurocholic acid.
- •
TCDCA: taurochenodeoxycholic acid.
- •
TDCA: taurodeoxycholic acid.
- •
TLCA: taurolithocholic acid.