Objective. This study evaluates hepatoprotective potential of Feronia limonia stem bark (FSB) extracts and fractions using experimental models.
Materials and methods. Activity levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and cell viability were evaluated in HepG2 cells treated with carbon tetrachloride (CCl4) in presence or absence of FL extracts or fractions. Also, plasma markers of hepatic damage, hepatic antioxidants, lipid peroxidation and histopathological alterations were assessed in rats treated with CCl4 alone or in combination with 200 or 400 mg/kg bodyweight (BW) of FSB-7 or 25 mg/kg BW of silymarin.
Results.In vitro co-supplementation of FSB extracts or fractions recorded varying degree of hepatoprotective potentials. Also, pre-supplementation of FSB methanolic extract (FSB-7) followed by CCl4 treatment significantly prevented hepatic damage and depletion of cellular antioxidants. Also, CCl4+ FSB-7 group showed minimal distortion in the histoarchitecture of liver and results were comparable to that of CCl4+ silymarin treated rats.
Conclusion. This inventory is the first scientific report on hepatoprotective potential of FSB methanolic extract.
Mammalian liver is a key organ associated with synthesis and secretion of digestive enzymes and elimination of toxic substances. Therefore toxicity of liver cells causes impairment of its function and associated physiological complications.1 Infectious pathogens and hepatotoxic chemicals are the main causative agents inducing impairment of liver function.2 Hence, natural remedies of herbal origin are considered to be an effective and safe alternative therapy as some of the synthetic drugs manifest hepatotoxicity due to their side effects. In Ayurveda, there are a variety of herbal treatments suggested for prevention of liver disorders.3 However, it is imperative to establish their efficacy and safety prior to development of newer medicaments acting against hepatic injury.
Feronia limonia (family Rutaceae, subfamily Aurantioideae), is commonly known as "kaitha" or wood apple and is widely distributed in deciduous and arid landscapes of several countries in South Asia.4Feronia limonia (FL) as a whole, or its parts such as unripe and ripe fruit, root, bark, trunk gum and leaves have a broad spectrum of traditionally established therapeutic potentials. Stem bark of FL has been reported to provide relief in cases of heartburns.5 Ethnic inhabitants of eastern India (Santals and Juangs) use stem bark extract of FL for treating asthma, bronchitis and skin ailments.6,7 Stem bark of FL has been reported to be rich in coumarins (bergapten, psoralen, demethylsubrosein, marmesin), tetranortriterpene (acidissimin), and flavanoids (Glucopyranoside, Urosolic Acid, (-)-(2S)-5,3“Dihydroxy-4”-methoxy-6”,6”-dimethylchromeno-(7,8,2”,3”)-flava-none).8-11
Recent studies from our lab have reported hepato-protective potential of FL leaf12 and root13 extracts. In continuation, present study assesses the hepato-protective potential of bioassay guided fractions of FL stem bark using in vitro and in vivo experimental models.
Materials and MethodsPlant materialFeronia limonia stem bark was collected during September-October 2008 from campus of The Maharaja Sayajirao University of Baroda, Vadodara, India. They were authenticated in the Department Botany and a voucher specimen (No. Pharmacy/FL/ 08-09/01/MJ) was deposited in the Pharmacy Department, The Maharaja Sayajirao University of Baroda, Vadodara, India.
Preparation of extractsThe stem barks were shade dried, powdered (500 g) and extracted three times with petroleum ether (3 x 1.5 L) in a soxhlet apparatus. The filtrates were then combined, filtered and concentrated to dryness in a rotary evaporator (Buchi-R-215, Germany) to obtain a crude petroleum ether extract (FSB-1). The remaining marc was then dried and exhaustively extracted at 6080 °C with methanol (3 x 1.5 L) in a soxhlet apparatus. The pooled extracts were then concentrated under vacuum to obtain methanolic extract (FSB-7). Hydro methanolic extract was made by addition of hot distilled water in methanol (1:1) and partitioned with chloroform (100 mL x 4) and combined chloroform fraction was then concentrated in vacuum to obtain a brown residue (4.5 g) (FSB-9). This residue was chromatographed over a Silica gel (60#120 mesh size) column and eluted with toluene followed by ascending concentrations of ethyl acetate and methanol. The resultant 7th fraction obtained from ethyl acetate-toluene (40:60) yielded an amorphous yellow powder on drying (FSB-11). The subsequent 10th fraction obtained from ethyl acetate-toluene (60:40) yielded yellow crystalline fraction (FSB-12).
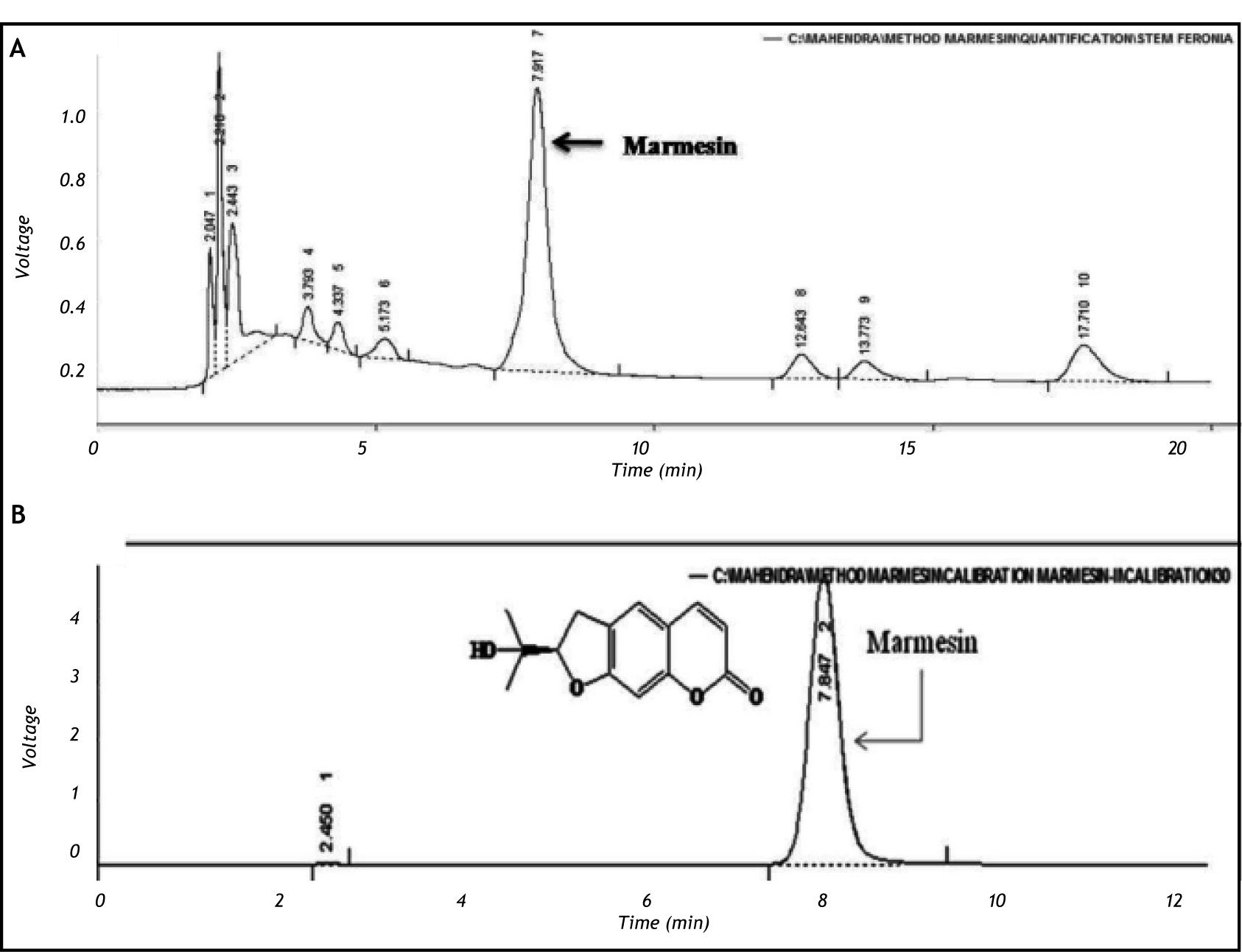
Standardization of FSB-S7 using marmesinPrecisely weighed samples (Feronia limonia stem bark) were extracted with methanol in an ultrasonic bath and filtered in 0.22 mm filter. An aliquot of 20 μL of sample was injected onto the high-pressure liquid chromatography (HPLC) column (XTerraTM RP C18 column, 4.6 x 250 mm, 5 mm particle size) and elution was carried out with methanol: water (1:1) at a flow-rate of 2 mL/min and the eluate was monitored at 280 nm. The procedure was repeated three times for each sample. The external standard calibration curve for marmesin was prepared with calibration solution with in the concentration range of 10-50 μg/mL. Each solution was prepared and injected three times and the curve was constructed (using Microsoft Office Excel 2007). The calculated concentration of marmesin was expressed in terms of percentage w/w.
In vitro hepatoprotective studies- •
Cell culture. Human liver hepatoma cells (HepG2) (obtained from National Centre for Cell Sciences, Pune, India) were seeded (1 x 105 cells/ T 25 Flask) and cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% antimicrobial-anti-mycotic solution for 24 h at 37°C with 5% CO2(Thermo scientific, forma II water jacketed CO2 incubator). Cells were sub-cultured every third day by trypsinization with 0.25 % Trypsin-EDTA solution. All the reagents were sterile filtered through 0.22 micron filter (Laxbro Bio-Medical aids Pvt. Ltd, Mumbai, India) prior to use for the experiment.
- •
Cytotoxicity assay. HepG2 cells (5.0 x 103 cells/well) were maintained in 96 well culture plate (Tarson India Pvt. Ltd) for 72 hr in presence of FSB-1, FSB-7, FSB-9, FSB-11 or FSB-12 at the concentrations of 10, 20, 100, 250, 500, 750 and 1,000 μg/mL. At the end of incubation period, 10 μL of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenylte-trazolium bromide (MTT; 5 mg/mL) was added to wells and the plate was incubated at 37°C for 4 h. At the end of incubation, culture media was discarded and wells were washed with phosphate buffer saline (Himedia Pvt. Ltd, Mumbai, India). Later, 150 uL of dimethyl sulphoxide was added to all the wells incubated for 30 min at room temperature with constant shaking. Absorbance was read at 540 nm using ELX800 universal microplate reader (Bio-Tek instruments, Inc, Winooski, VT) and subsequently percentage cell viability was calculated.14
- •
CCL4 induced hepatotoxicity assay in HepG2 cells. HepG2 cells (5.0 x 103 cells/well) were maintained in culture media containing 1% carbon tetrachloride (CCl4) in presence or absence of FSB-1, FSB-7, FSB-9, FSB-11, FSB-12 or silymarin at the concentrations of 10, 20, 50, 100, 200 μg/mL for 24 h. Later, supernatants were removed and activity levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined. Cell viability assay was performed in the adherent cells as per the modified method described in our previous report.12
Male Wistar albino rats (obtained from Zydus Research Centre, Ahmedabad, India) were housed and maintained in clean polypropylene cages and fed with laboratory chow (Pranav agro ltd, India). Experiments were carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India and approved by the institutional animal ethical committee (IAEC) of Department of Pharmacy, Faculty of Technology and Engineering, The Maharaja Sayajirao University of Baroda, Vadodara, INDIA.
Acute toxicity studiesAcute oral toxicity study was conducted using the limit test procedure as per the organization for economic co-operation and development (OECD) test guidelines on acute oral toxicity test 401.15 Thirty two Wistar rats of either sex were divided into four groups (n = 8) and were orally administered with a single dose of 1,000 mg, 2,000 mg, 3,000 mg or 5,000 mg/kg BW of FSB-7. Animals were observed for possible behavioral changes such as tremors, convulsions, sleep, altered feeding, salivation, altered somato-motor activities and diarrhea for 7 days post treatment.
In vivo hepatoprotective studiesThirty rats were randomly divided into 5 groups of 6 each. Group-I (CON) served as normal control and was orally given 0.5% carboxy methyl cellulose (CMC) solution daily for 7 days. Group-II (CCl4) were given 0.5% CMC solution (0.1 mL) daily for 7 days. Group-III and IV (CCl4 + FSB-7A and CCl4 + FSB-7B) were orally given 200 or 400 mg/kg of FSB-7 once daily for 7 days respectively. Group-V (CC14 + SYL) were orally given silymarin (25 mg/kg) once daily for 7 days.16 Groups II, III, IV and V were injected with a single dose of 0.5 mL/kg CCl4 (i.p.) mixed with olive oil (1:1) on 7th day of the study16 whereas, group I received equal volume of olive oil.
On the 8th day, blood sample were collected via retro-orbital sinus puncture under mild ether anesthesia and plasma was separated for biochemical analysis. Later, animals were sacrificed by cervical dislocation under mild ether anesthesia and liver was excised and stored at-80 °C for further estimations.
- •
Plasma biochemical assays. Plasma AST, ALT, alkaline phosphatase (ALP), total bilirubin and total protein were assayed using commercially available kits (Reckon diagnostics, Baroda, India).
- •
Hepatic antioxidants and lipid peroxidation. Liver of control and treated animal were excised, weighed and homogenized in chilled (4 °C) tris buffer (10 mM, pH 7.4) at a concentration of 10% (w/v). The homogenates were then centrifuged at 10,000 x g at 0 °C for 20 min in high speed cooling centrifuge. Supernatant was used for the assay of superoxide dismutase (SOD),17 catalase (CAT),18 reduced glutathione (GSH),19 total protein20 and lipid peroxidation (LPO)21 whereas, ascorbic acid (AA) content was determined in sediment.22
- •
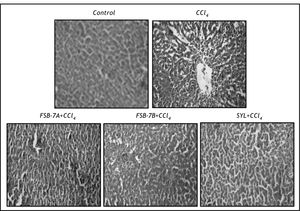
Histopathological evaluations of liver. Liver samples were fixed in 4% buffered paraformaldehyde, dehydrated in graded alcohol series and embedded in paraffin wax. Five ¿u.m thick sections were cut and stained with hematoxyline and eosin and examined for gross structural changes. Photographs were taken using Canon power shot S72 digital Camera (200 X).
Data was analyzed for statistical significance using one way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test and results were expressed as mean ± SEM using Graph Pad Prism version 3.0 for Windows, Graph Pad Software, San Diego California USA.
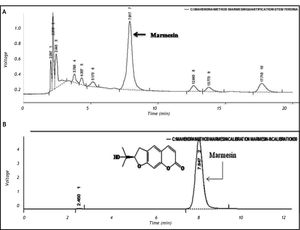
ResultsQuantitative analysis of marmesin by HPLCThe calibration curve was prepared with marmesin and was found to be linear (R2 = 0.988) in the concentration range (10-50 μg/mL). The concentration of marmesin in FSB-7 was found to be 0.03112% w/w. The chromatogram obtained for FSB-7 revealed multiple peaks including that of marmesin (Figure 1).
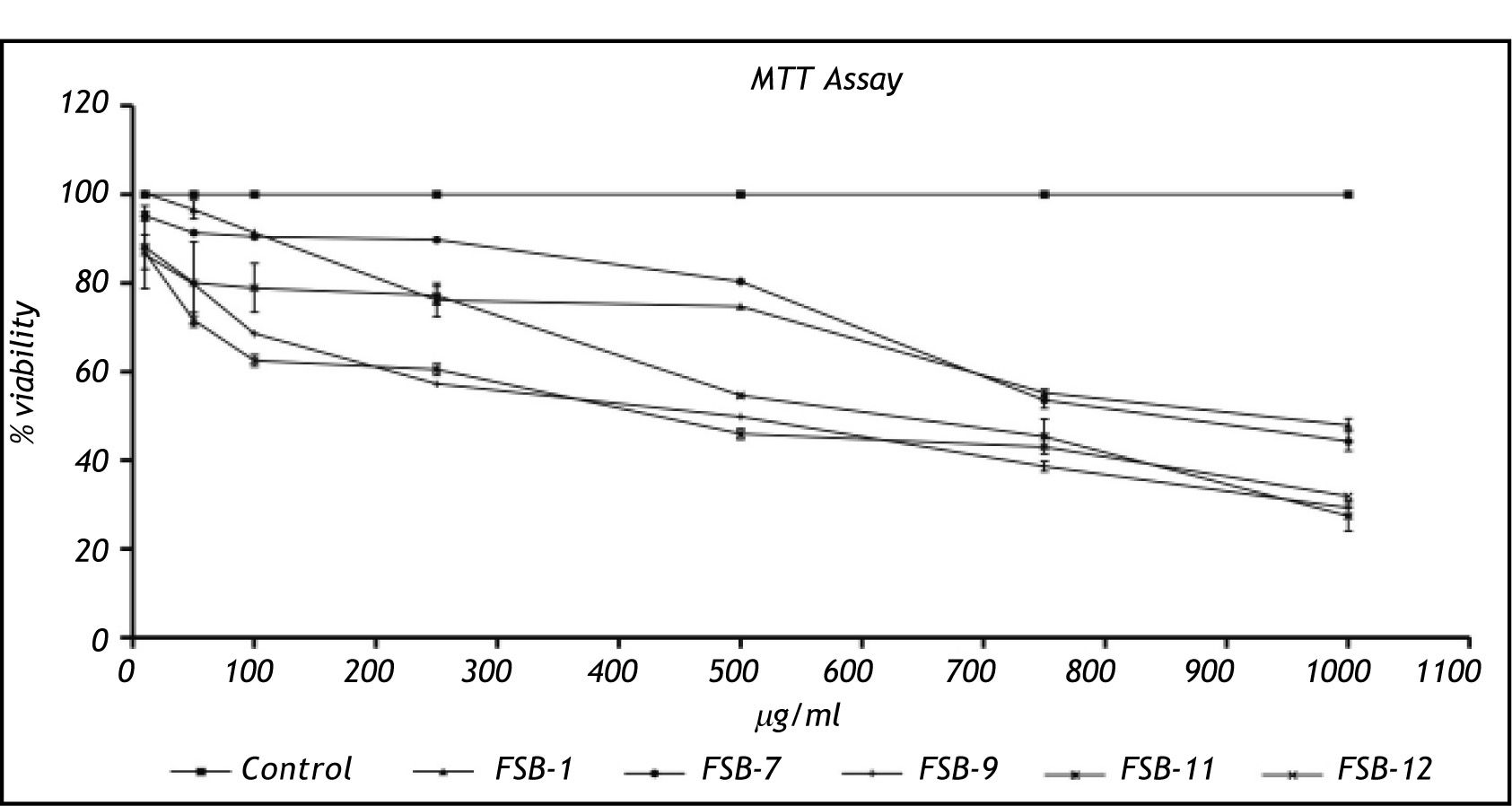
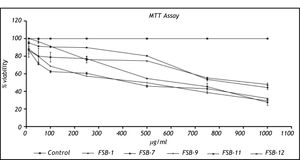
Cytotoxicity studiesCytotoxicity assessment of FSB-1, FSB-7 and FSB-11 revealed an identical pattern of cytotoxicity that was marked by > 50% viable cells at 250 μg/mL dose. However FSB-9 and FSB-12 appeared to be relatively more toxic because they showed < 50 % viability at 250 μg/mL dose (Figure 2).
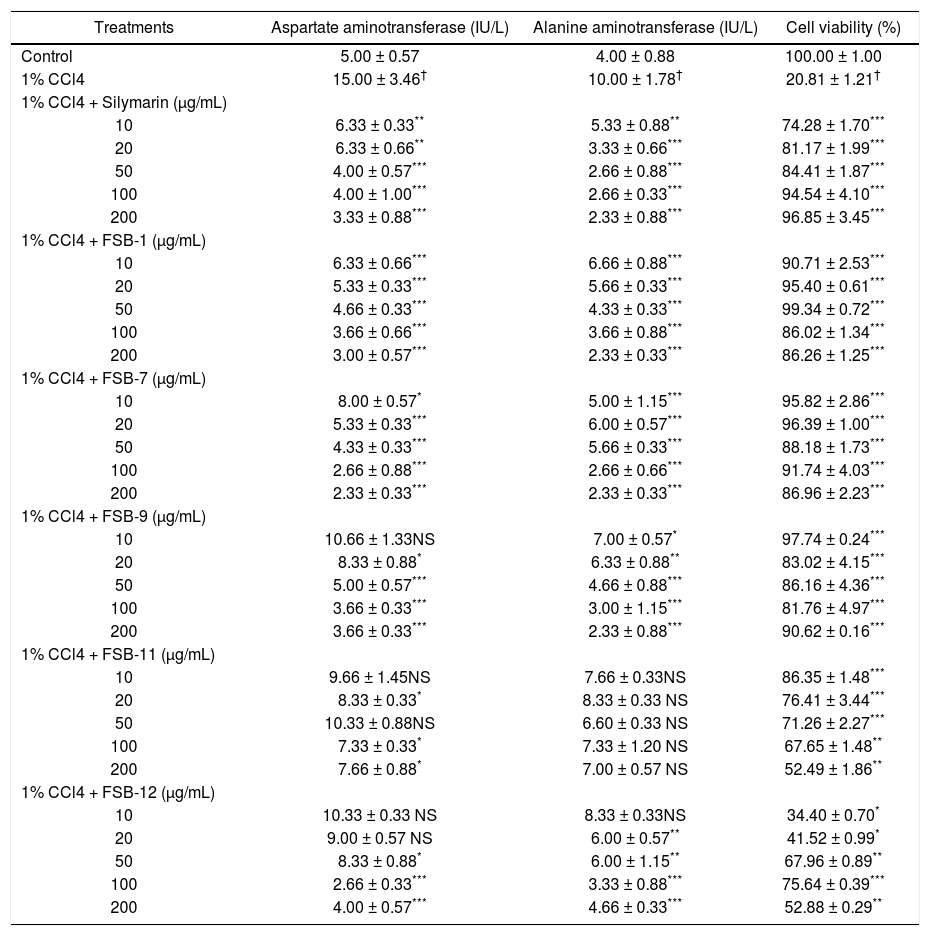
In vitro hepatoprotective studiesActivity levels of AST and ALT in cell supernatants recorded a significant increment in cells treated with 1% CCl4. However, co-supplementation of FSB-1, FSB-7 or FSB-9 recorded a nonlinear dose dependent decrease in activity levels of AST and ALT that were comparable to dose dependent decrease observed in CCl4 + silymarin treated groups (table 1). Similar set of results were observed in cell viability of HepG2 cells (Table 1).
Effect of Feronia limonia extracts, fractions and silymarin on carbon tetrachloride (CCl4) induced hepatotoxicity.
| Treatments | Aspartate aminotransferase (IU/L) | Alanine aminotransferase (IU/L) | Cell viability (%) |
|---|---|---|---|
| Control | 5.00 ± 0.57 | 4.00 ± 0.88 | 100.00 ± 1.00 |
| 1% CCl4 | 15.00 ± 3.46† | 10.00 ± 1.78† | 20.81 ± 1.21† |
| 1% CCl4 + Silymarin (μg/mL) | |||
| 10 | 6.33 ± 0.33** | 5.33 ± 0.88** | 74.28 ± 1.70*** |
| 20 | 6.33 ± 0.66** | 3.33 ± 0.66*** | 81.17 ± 1.99*** |
| 50 | 4.00 ± 0.57*** | 2.66 ± 0.88*** | 84.41 ± 1.87*** |
| 100 | 4.00 ± 1.00*** | 2.66 ± 0.33*** | 94.54 ± 4.10*** |
| 200 | 3.33 ± 0.88*** | 2.33 ± 0.88*** | 96.85 ± 3.45*** |
| 1% CCl4 + FSB-1 (μg/mL) | |||
| 10 | 6.33 ± 0.66*** | 6.66 ± 0.88*** | 90.71 ± 2.53*** |
| 20 | 5.33 ± 0.33*** | 5.66 ± 0.33*** | 95.40 ± 0.61*** |
| 50 | 4.66 ± 0.33*** | 4.33 ± 0.33*** | 99.34 ± 0.72*** |
| 100 | 3.66 ± 0.66*** | 3.66 ± 0.88*** | 86.02 ± 1.34*** |
| 200 | 3.00 ± 0.57*** | 2.33 ± 0.33*** | 86.26 ± 1.25*** |
| 1% CCl4 + FSB-7 (μg/mL) | |||
| 10 | 8.00 ± 0.57* | 5.00 ± 1.15*** | 95.82 ± 2.86*** |
| 20 | 5.33 ± 0.33*** | 6.00 ± 0.57*** | 96.39 ± 1.00*** |
| 50 | 4.33 ± 0.33*** | 5.66 ± 0.33*** | 88.18 ± 1.73*** |
| 100 | 2.66 ± 0.88*** | 2.66 ± 0.66*** | 91.74 ± 4.03*** |
| 200 | 2.33 ± 0.33*** | 2.33 ± 0.33*** | 86.96 ± 2.23*** |
| 1% CCl4 + FSB-9 (μg/mL) | |||
| 10 | 10.66 ± 1.33NS | 7.00 ± 0.57* | 97.74 ± 0.24*** |
| 20 | 8.33 ± 0.88* | 6.33 ± 0.88** | 83.02 ± 4.15*** |
| 50 | 5.00 ± 0.57*** | 4.66 ± 0.88*** | 86.16 ± 4.36*** |
| 100 | 3.66 ± 0.33*** | 3.00 ± 1.15*** | 81.76 ± 4.97*** |
| 200 | 3.66 ± 0.33*** | 2.33 ± 0.88*** | 90.62 ± 0.16*** |
| 1% CCl4 + FSB-11 (μg/mL) | |||
| 10 | 9.66 ± 1.45NS | 7.66 ± 0.33NS | 86.35 ± 1.48*** |
| 20 | 8.33 ± 0.33* | 8.33 ± 0.33 NS | 76.41 ± 3.44*** |
| 50 | 10.33 ± 0.88NS | 6.60 ± 0.33 NS | 71.26 ± 2.27*** |
| 100 | 7.33 ± 0.33* | 7.33 ± 1.20 NS | 67.65 ± 1.48** |
| 200 | 7.66 ± 0.88* | 7.00 ± 0.57 NS | 52.49 ± 1.86** |
| 1% CCl4 + FSB-12 (μg/mL) | |||
| 10 | 10.33 ± 0.33 NS | 8.33 ± 0.33NS | 34.40 ± 0.70* |
| 20 | 9.00 ± 0.57 NS | 6.00 ± 0.57** | 41.52 ± 0.99* |
| 50 | 8.33 ± 0.88* | 6.00 ± 1.15** | 67.96 ± 0.89** |
| 100 | 2.66 ± 0.33*** | 3.33 ± 0.88*** | 75.64 ± 0.39*** |
| 200 | 4.00 ± 0.57*** | 4.66 ± 0.33*** | 52.88 ± 0.29** |
No mortality was recorded in animals that were orally administered up to 5,000 mg/kg of FSB-7. Also there were no adverse behavioral changes, diarrhea, salivation or food aversion. No major changes in the gross weight of organs (data not shown) could be observed following FSB-7 (5,000 mg/kg) administration.
Hepatic antioxidants and lipid peroxidationSignificant decrement in activity levels of hepatic enzymatic (SOD and CAT) and non-enzymatic (GSH and AA) antioxidants were recorded in CCl4 treated rats. FSB-7 pre-treatment was able to prevent CCl4 induced depletion of hepatic antioxidant. A dose of 500 mg/kg BW of FSB-7 was found to be most efficient and comparable to SYL + CCl4 treated group. Hepatic LPO levels were significantly high in CCl4 treated groups. But, LPO level were similar in SYL, FSB-7 treated groups and comparable to control group (Table 2).
Effect of Feronia limonia stem nark methanolic extract (FSB-7) and silymarin (SYL) on hepatic antioxidants and lipid peroxidation during carbon tetrachloride (CCl4) induced hepatotoxicity.
| Experimental group | Superoxide dismutase (U/min/mg protein) | Catalase (U/min/mg protein) | Reduced glutathione (mg/g) | Ascorbic acid (mg/g) | Lipid peroxidation (nmol of MDA formed/mg protein |
|---|---|---|---|---|---|
| Control | 62.21 ± 4.31 | 33.45 ± 1.56 | 7.00 ± 0.45 | 4.21 ± 0.25 | 2.77 ± 0.39 |
| 0.5 mL/kg CCl4 | 25.53 ± 1.88† | 16.14 ± 1.99† | 2.45 ± 0.21† | 1.89 ± 0.22† | 5.67 ± 0.21† |
| SYL (25 mg/kg) + | |||||
| 0.5 mL/kg CCl4 | 52.11 ± 4.89*** | 31.11 ± 2.11** | 4.88 ± 0.34*** | 3.54 ± 0.17*** | 2.41 ± 0.29*** |
| FSB-7 (200 mg/kg) + | |||||
| 0.5 mL/kg CCl4 | 45.00 ± 4.11*** | 22.33 ± 0.53* | 3.64 ± 0.02* | 2.86 ± 0.04** | 3.31 ± 0.02** |
| FSB-7 (400 mg/kg) + | |||||
| 0.5 mL/kg CCl4 | 51.00 ± 5.01*** | 27.00 ± 0.35** | 4.23 ± 0.25*** | 3.23 ± 0.05*** | 2.92 ± 0.01*** |
Significantly higher levels of AST, ALT, ALP and total bilirubin were recorded in CCl4 treated rats whereas; the total protein content in plasma was significantly reduced. However, FSB-7 treated groups prevented CCl4 induced elevation in plasma markers of hepatic damage and prevented CC14 induced decrement in plasma protein. However, FSB-7 treatment prevented CC14 induced changes in these parameters with 400 mg/kg BW dose being the most efficient (Table 3).
Effects of Feronia limonia stem nark methanolic extract (FSB-7) and silymarin (SYL) on plasma levels of hepatic injury markers during carbon tetrachloride (CCl4) induced hepatotoxicity.
| Treatments | Alanine aminotransferase (U/L) | Aspartate aminotransferase (U/L) | Alkaline phospatase (U/L) | Total bilirubin (mg/dL) | Total protein (g/dL) |
|---|---|---|---|---|---|
| Control | 46.11 ± 2.99 | 84.66 ± 9.00 | 1.69 ± 0.13 | 1.40 ± 0.22 | 8.00 ± 0.84 |
| 0.5 mL/kg CCl4 | 140.41 ± 4.23† | 280.16 ± 12.41† | 3.78 ± 0.31† | 3.88 ± 0.42† | 4.54 ± 0.94† |
| SYL (25 mg/kg) + | 52.41 ± | 92.11 ± | 1.45 ± | 1.51 ± | 7.00 ± |
| 0.5 mL/kg CCl4 | 3.00*** | 12.56*** | 0.23* | 0.21*** | 0.71*** |
| FSB-7(200 mg/kg) + | 71.66 ± | 142.16 ± | 2.52 ± | 2.50 ± | 6.26 ± |
| 0.5 mL/kg CCl4 | 2.59** | 13.04** | 0.10* | 0.25** | 0.02** |
| FSB-7(400 mg/kg) + | 62.83 ± | 121.16 ± | 2.25 ± | 2.07 ± | 6.55 ± |
| 0.5 mL/kg CCl4 | 4.20*** | 11.76*** | 0.21* | 0.02** | 0.03** |
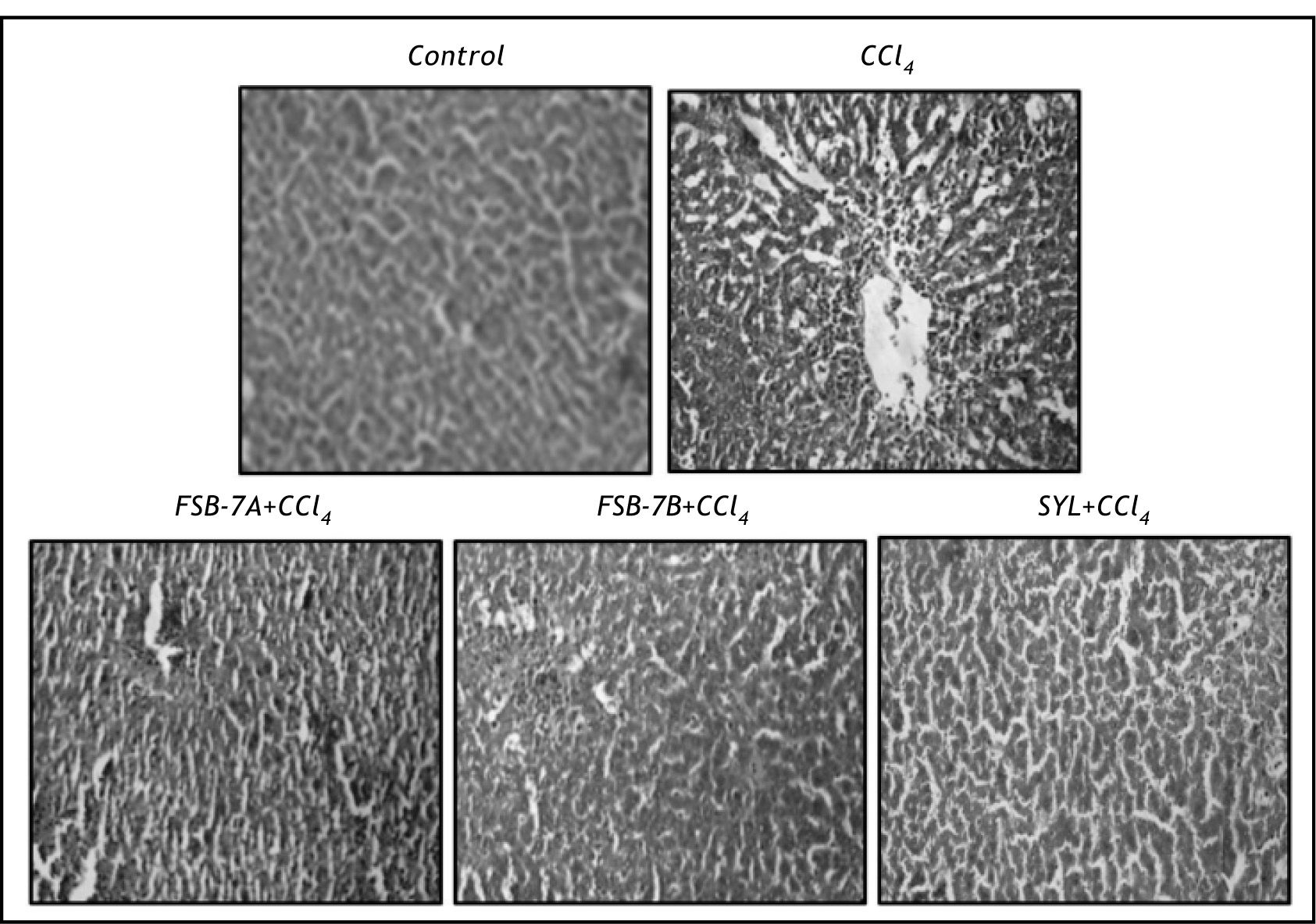
Hepatocyte vacuolation, centrilobular necrosis and nuclear condensation were evident in hematoxyline and eosin stained liver section of CCl4 group (Figure 3). These cellular changes were greatly reduced in FSB-7 treated group with higher dose (400 mg/kg) recording mild necrosis and relatively healthy hepatocytes that were comparable to the control and SYL treated groups (Figure 3).
DiscussionPrior to the therapeutic use of any herbal extract, it is imperative to perform a cytotoxicity assay. This is because crude extract of many herbs have been shown to be non-toxic but some of its bio-assay guided fraction or pure compound may show toxicity.23 In the present study, cytotoxicity evaluation was performed with the dose range of 10-1,000 Ug/mL of FL extracts and fractions (FSB-1, FSB-7, FSB-9, FSB-11and FSB-12). Since, all the extract/fractions tested for cytotoxicity assay showed > 50% cell viability at 250 μg/mL dose, they were used for in vitro hepatoprotective study.
HepG2 cell line is a popular in vitro model where in, cells are subjected to CCl4 induced toxicity and subsequent amelioration using a desired herbal extract or compound in question.24 In the present study, various extracts/fractions of FL (FSB-1, FSB-7, FSB-9, FSB-1 land FSB-12) where subjected to scrutiny for assessing their hepatoprotective potentials. Results revealed that FSB-7 and FSB-9 imparted hepatoprotection and the same was evident in form of a dose dependent decrement in activity levels of AST and ALT and improved cell viability. Based on these observations and toxicity study, FSB-7 was chosen for subsequent in vivo analysis of its hepatoprotective potential.
It has been reported that, hepatic cytochrome P450 dependent monooxygenases converts CCl4 into highly reactive trichloromethyl (CCl3) radicals. These CCl3 radicals then, alkylate the cellular protein and abstract an electron from polyunsaturated fatty acid of cellular membrane to initiate lipid peroxidation.16 These lipid peroxides create severe intracellular oxidative stress and depletion of cellular antioxidants resulting in gross damages to the DNA, cell membrane and mitochondria.16
The non-enzymatic antioxidant, GSH is one of the most abundant tripeptides, widely distributed in liver cells. Its functions are mainly concerned with the removal of free radical species such as hydrogen peroxides, superoxide radicals, alkoxy radicals and maintenance of membrane protein thiols.25 The body has an effective defense mechanism to prevent and neutralize the free radical-induced damage. This is accomplished by a set of endogenous antioxidant enzymes such as SOD and CAT. These enzymes constitute a mutually supportive team of defense against reactive oxygen species (ROS). Superoxide dismutase represents the first line of defense against free radicals and catalyze dismutation of superoxide radical to oxygen and hydrogen peroxide.26 Catalase enzyme catalyzes the decomposition of hydrogen peroxide and other peroxides (e.g., lipid peroxides in cell membranes) into oxygen and water, using reduced glutathione as substrate.26 In consistence with the previous reports,26,27 we recorded elevated indices of LPO, and decrement in GSH, AA, SOD and CAT in CCl4 treated rats. However, FSB-7 (400 mg/kg) was capable of preventing oxidative stress and depletion of tissue antioxidants. Presently recorded minimum depletion of antioxidants and lowered LPO indices in FSB-7 treated rats is due to high content of marmesin that scavenges free radicals produced due to CCl4 treatment.12
Microscopic evaluation of liver sections of control rats depicted unaltered cellular architecture with distinct hepatic cells, sinusoidal spaces and a central vein. Whereas, liver section of CCl4 treated rats showed loss of cellular boundaries, infiltration of inflammatory cells, cytoplasmic vaculation, fatty change, centri-lobular necrosis and severe collagen deposition. These observations corroborate other reports on hepatic damage following CCl4 treatment. 28-29 FSB-7 supplementation to CCl4 treated rats minimized these set of changes in a dose dependent manner and the results were comparable to that of control and CCl4 + SYL groups. These results are in accordance with other reports on protective role of herbal extract against CCl4 induced hepatic damage30 and justifies presently recorded biochemical and microscopic changes.
Carbon tetrachloride induced hepatocyte damage or necrosis results in the leakage of marker enzymes of liver function and hence, elevations in plasma levels of AST, ALT, ALP, bilirubin and total protein are indicators of hepatotoxicity.31 Presently recorded elevated levels of AST, ALT, ALP, bilirubin and total protein in the CCl4 treated rats are in accordance with previous reports.32,33 The restoration of these parameters to near normal levels in CCl4 + FSB-7 treated group indicates at the ability of FSB-7 in preventing cellular damage and subsequent leakage of intracellular enzymes and bilirubin. The same stands well correlated with histopathological evaluations. It is assumed that FSB-7 imparts hepatoprotection against free radicals induced oxidative damage and stabilizes the hepatic cells against further degradation.
ConclusionThis study establishes the hepatoprotective potential of FL stem bark and the same is attributable to the presence of marmesin in FSB-7. It may be required a detailed investigation at the clinical level to translate the present findings into developing a new hepatoprotective herbal medicine.
AcknowledgementThe authors are thankful to Dr. Geeta. S. Padate, Head, Department of Zoology for her encouragement and providing necessary permission.
Conflict of InterestAuthors declare no conflict of interest