Systemic symptoms such as fever and fatigue are non-specific manifestations spanning from inflammation to neoplasia. Here we report the case of a 34 year-old man who presented with systemic symptoms for four months. CT-scan and MRI revealed a 3.4 cm arterialized hepatic lesion and a 7 cm paraduodenal mass. Surgical resection of both lesions and histological examination revealed an inflammatory hepatocellular adenoma and a unicentric plasma cell type of Castleman disease. Moreover, a diffuse AA amyloid deposition in the liver was observed. Resection of both lesions was associated with an improvement of the symptoms. To our knowledge, this is the first report of a synchronous presentation of a unicentric plasma cell type of Castleman disease, inflammatory hepatocellular adenoma and AA amyloidosis.
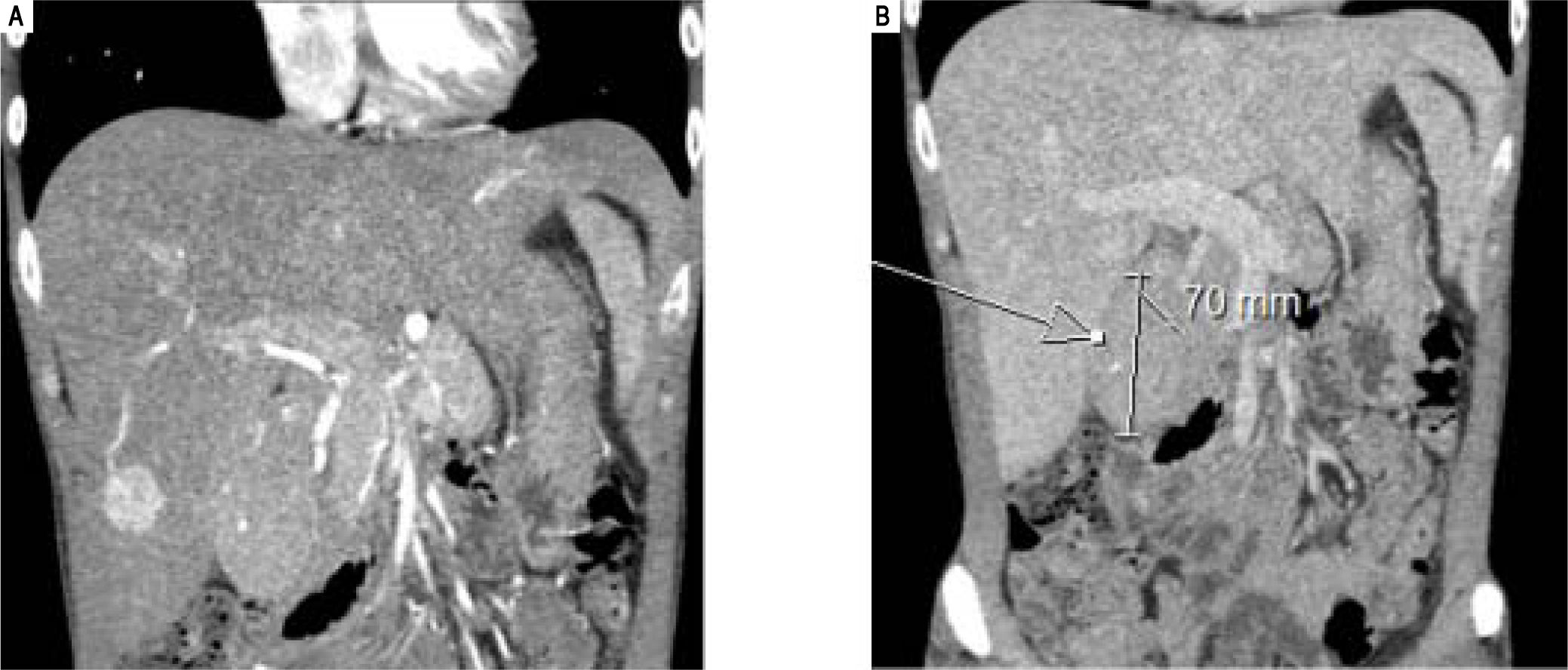
A 34 year-old male presented with a four months history of systemic symptoms including anorexia, night sweating, fever and fatigue. Laboratory evaluation showed low haemoglobin (104 g/L), high platelets (691*109/L), increased alkaline phosphatase (211 IU/L) and gamma-glutamyl transferase (92 IU/L) associated to low albumin (27 g/L) in addition to high serum levels of C reactive protein (CRP) (162.9 mg/L). HIV status was negative. CT and MRI scans revealed two lesions: a 3.4 cm, well-circumscribed, arterialised mass in the inferior part of the right liver arising in a background of hepatomegaly and a paraduodenal mass measuring 7 cm in maximum dimension (Figure 1).
Histological FindingsThe core needle biopsy from the liver lesion showed bland hepatocytes associated with sinusoidal dilatation, a focal lymphocytic infiltrate and unpaired arteries. Bile ductule-like structures not associated with other portal vascular structures were also present. Serum Amyloid A (SAA) showed diffuse immunostaining in hepatocytes, suggesting an inflammatory hepatocellular adenoma (i-HCA). A fine needle aspiration biopsy of the paraduodenal mass revealed only lymphocytes with no evidence of malignancy.
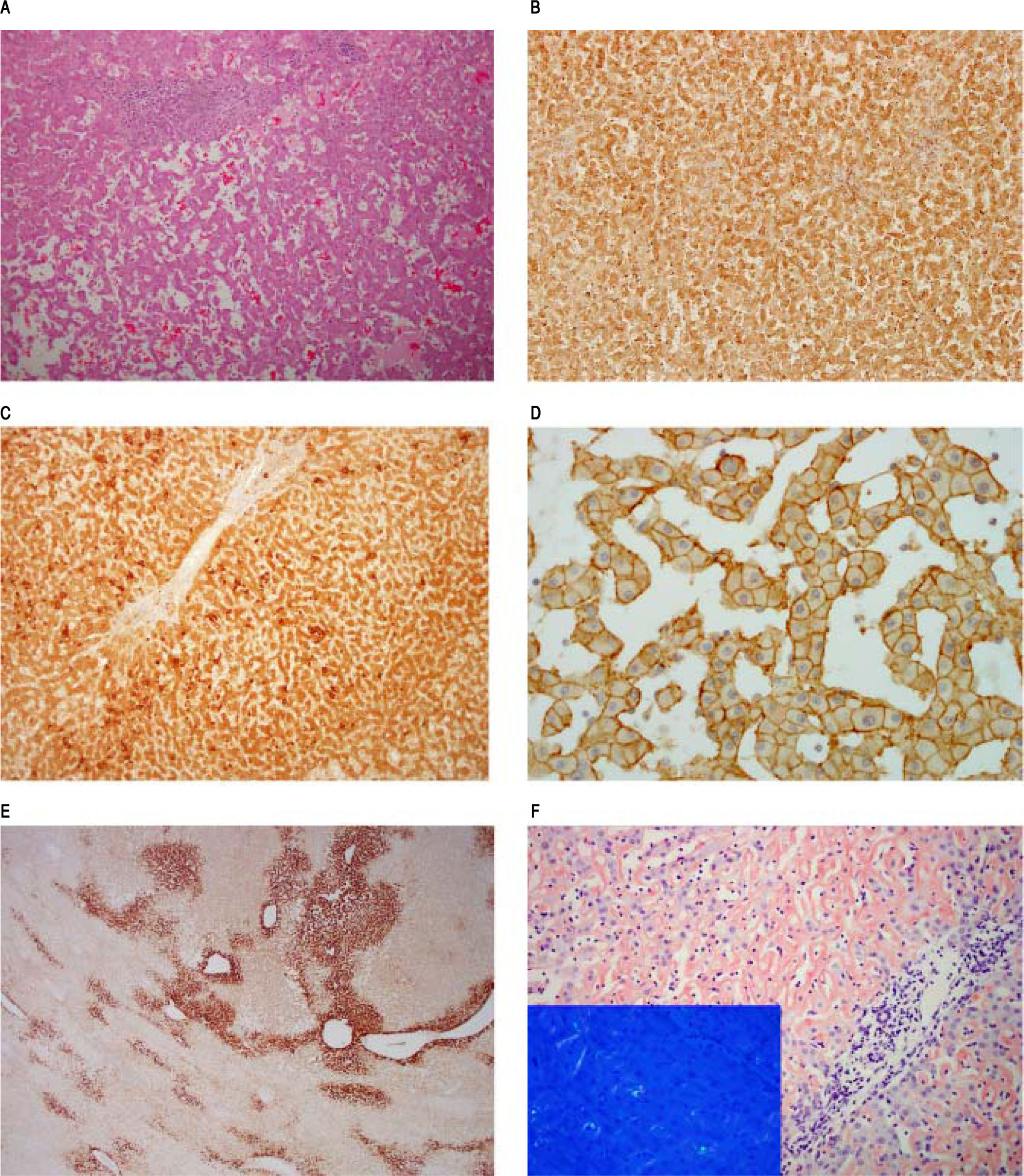
Hepatic segmentectomy (segment VI) was subsequently performed and histological examination revealed a proliferation of bland hepatocytes, disposed in cords; occasionally several cells thick, with ectasic sinusoid-like spaces (Figure 2A). On immunohistochemical grounds, CRP showed strongly immunostaining both in tumor and perilesional liver (Figure 2B) as well as SAA (Figure 2C) and liver fatty acid binding protein-1 (not shown). Beta-catenin immunostain exhibited only membranous expression in lesional hepatocytes (Figure 2D) without any diffuse immunostaining of glutamine synthetase (Figure 2E). The overall features were consistent with an i-HCA.1
Inflammatory hepatocellular adenoma, characterized by a bland hepatocytes proliferation, with ectasic sinusoids, magnification 100x. B. CRP immunostaining, magnification 100x. C. SAA immunostaining, magnification 100x. D. B-catenin immunostaing, magnification 400x. E. Glutamine synthetase immunostaining, magnification 40x. F. Congo Red stain in the background liver. Inset shows apple-green birefringence under polarized light on Congo Red stain.
The perilesional liver showed no significant fibrosis on Sirus red stain, and a mild and non-specific portal-tract inflammatory cell infiltrate. Plasma cells were not identified. Within the parenchymal sinusoidal space, an amorphous, eosinophilic material, was identified mainly in the centrilobular regions. On special histochemical grounds, amyloid deposition was confirmed; given the apple-green birefringence under polarized light on Congo Red stain (Figure 2F). These deposits stained for SAA and both for K-and A-light chains (not shown); the latter best regarded as a sign of nonspecificity.
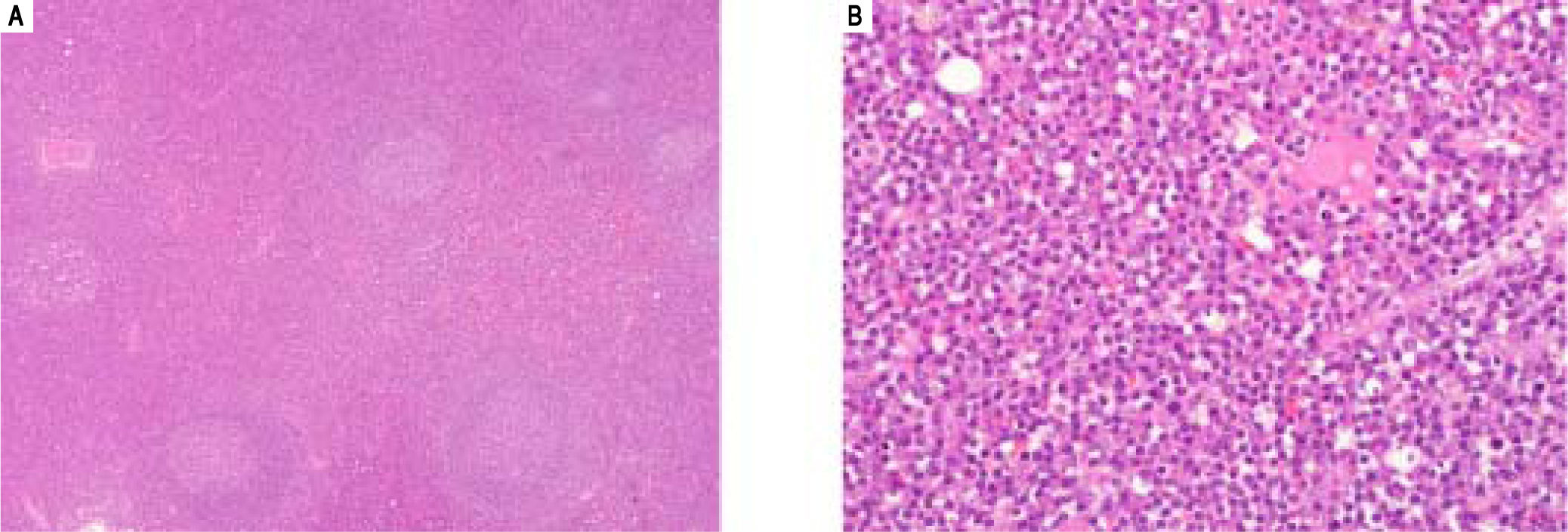
Simultaneously to the liver resection the paraduodenal mass and a portal lymph node were removed. Both displayed similar appearances: variably-sized lymphoid follicles with germinal centers, containing tingible bodies macrophages and occasionally a hyaline material arranged in a globular pattern. This did not stain for Congo Red, while light chain-specific immunostainings were noncontributory due to heavy background staining. The germinal centers were surrounded by concentric rings of lymphocytes with sheets of polytypic plasma cells seen in the interfollicular areas (Figure 3). HHV-8 immunostain was negative. These findings were consistent with a unicentric plasma cell type of Castleman disease (CD). The possibility of a multi-centric CD (MCD) was ruled out by a PET-scan.
Within five months after surgery, CRP decreased to within the normal ranges (from: 162.9 mg/L to < 2.0 mg/ L) and the systemic symptoms improved. Last follow-up, 3 years after surgery, the patient was well with no sign of i-HCA recurrence or chronic liver disease.
DiscussionI-HCA is a variant of HCA associated with systemic inflammatory conditions,1,2 obesity and high alcohol intake and may be associated with JAK/STAT3 pathway activation due to a mutation of the gp130 gene encoding for IL6ST (60%), Fyn-related kinase (FRK) (10%), STAT3 (5%), GNAS (5%) or JAK1 (1% of cases).3 Although the large majority of HCA develop in a non-cirrhotic liver, a recent study suggested that i-HCA could arise in a background of advanced chronic liver disease.4
Castleman disease is a rare lymphoproliferative disorder classified into two clinical entities (unicentric and multicentric form) and three pathological types (hyaline-vascular, plasma cell, and mixed cell type). MCD encompasses a spectrum of disorders that give rise to overlapping clinicopathological manifestations driven by proinflammatory hypercytokinaemia; Kaposi sarcoma-associated herpes virus/human herpes virus-8 (KSHV/HHV8)-related and -unrelated (idiopathic) multicentric CD forms.5’6 Although the current knowledge regarding the pathogenesis of lesions within the MCD spectrum is still evolving, hypercytokinaemia, often including IL-6, seems to play a pivotal role in the lymph node enlargement and characteristic histological features.6 Unicentric CD is further subclassified as hyaline vascular type (90%) and plasma cell type (10%). The latter appears to be almost always associated with systemic symptoms. i.e. fever, night sweats, fatigue, weight loss, splenomegaly, anemia and hypergammaglobulinemia, and abnormal laboratory findings.5
Amyloidosis results from the misfolding of extracellular protein and subsequent tissue deposition leading eventually to organ impairment. To date, 31 known extracellular fibril proteins are well documented in human.7 SAA is an acute phase protein, released by hepatocytes during inflammation under the control of several cytokines, in particular IL-6.8 SAA has been involved in hepatic amyloidosis; albeit observed at different frequencies ranging from 4% up to 44%.9’10
More than 50 reports of amyloidosis associated with CD have been published thus far in the medical scientific literature including hepatic amyloid deposition.11-15 Of note, Morita-Hoshi, et al.11 reviewed 45 cases of systemic amyloidosis related to CD and showed that:
- •
The plasma cell type was the most common histology in patients either with unicentric or multicentric disease complicated with amyloidosis.
- •
Almost all cases (95%) in which the type of amyloidosis was confirmed had the AA type.
- •
Surgical resection of the tumor mass was effective as a therapeutic modality in the majority of cases with unicentric CD.
The association between hepatic and/or systemic amyloidosis and hepatocellular adenomas has been rarely reported.16-19 Unfortunately, these cases were published prior to the classification of HCA,20 limiting comparison with the current case. The present case highlights the synchronous manifestation of three diseases potentially related to IL-6 overproduction by CD and/or IL-6/STAT3 pathway dysfunction. Long-term chronic inflammatory syndrome, mediated by high levels of IL-6, could stimulate hepatic proliferation leading to hepatomegaly14,21 and/ or promote tumor growth, i.e. hepatocellular carcinoma22 and i-HCA, as described herein. In particular, Chun, et al.22 described a unique case of synchronous presentation of retroperitoneal CD and hepatocellular carcinoma in a healthy 34-year-old man.
Proinflammatory hypercytokinaemia might contribute to the development of AA amyloidosis in some patients; given the IL-6 stimulation of hepatocytes and the resultant SAA overproduction.23,24 In keeping with other cases of unicentric CD,11 the surgical lymph node excision was effective leading to complete remission of the systemic inflammatory state (normalized levels of CRP). Nevertheless, liver biopsy has not been performed in our case to confirm a potential decrease in amyloid deposition, as previously documented on histopathological and/or imaging grounds.14,25-28
To the best of our knowledge, this is the first case of a synchronous presentation of a unicentric plasma cell variant of CD, inflammatory hepatocellular adenoma and AA amyloidosis. Whether this rare manifestation is attributed either to IL-6 secreting lymph nodes or hypercytokinaemia and/or IL-6/STAT3 pathway perturbations outside the lymph nodes7 remains elusive. The simultaneous resection of the enlarged lymph nodes and the inflammatory hepatocellular adenoma led to remission of the systemic inflammatory state and gradual improvement of the clinical symptoms.
Abbreviations- •
AA: myloid A.
- •
CD: Castleman disease.
- •
CRP: C eactive protein.
- •
i-HCA: inflammatory hepatocellular adenoma.
- •
MCD: Multicentric Castleman disease.
- •
SAA: serum amyloid A.
The authors declares that there is no conflict of interest regarding the publication of this article.
Financial SupportCDV is supported by the Nuovo-Soldati Foundation.
AcknowledgmentsThe authors would like to thank Dr. S. Pomplun for her expert opinion on the lymph node pathology.