The HCV protease inhibitor telaprevir associated with peginterferon-alpha and ribavirin, was widely used in the recent past as standard treatment in HCV genotype-1 infected patients. Telaprevir improves the sustained virology response rates, but at the same time increases the frequency of adverse cutaneous reactions. However, mechanisms through which telaprevir induces cutaneous lesions are not yet defined. A 50-year-old woman, affected by HCV genotype 1b, was admitted to our Department for a telaprevir-related severe cutaneous eruptions, eight weeks after starting a triple therapy (telaprevir associated with Peginterferon-alpha and ribavirin). Mechanisms of cutaneous reactions were investigated by skin tests with non-irritating concentrations of telaprevir and by activating in vitro T lymphocyte with different concentrations. Immediate and delayed responses to skin testing were negative, but the drug-induced lymphocytes activation was significantly higher as compared to patient’s baseline values and to parallel results obtained in three healthy subjects (p < 0.05). In conclusion, adverse cutaneous reactions of our patient were caused by a telaprevir-induced T-cell dependent immune mechanism.
The recent development of direct-acting antiviral agents (DAAs), which inhibit the replication of genotype-1 of hepatitis C virus (HCV), has improved sustained virology response rates of treated-naïve and experienced patients. Telaprevir was widely used in the recent past, although currently it is not frequently used, due to the availability of new DAAs. The combined use of telaprevir with Peginterferon (PEG-IFN)-alpha and ribavirin (RBV) for therapy of genotype-1 HCV chronically infected patients improved antiviral efficacy, but led to a significant increase in frequency and severity of adverse cutaneous reactions (ACRs) compared to PEG-IFN with RBV treatment.1–3 Indeed, while ACRs associated with dual therapy consist of pruritus, skin xerosis and eczematous like lesions, that are more frequent at skin-folds, the additional use of telaprevir induced an increase in the number of ACRs, which were severe in 6% of cases; in fact 3 patients suffered Stevens-Johnson syndrome (SJS) and in other 11 patients was observed a Drug Reaction with Eosinophilia and Systemic symptoms (DRESS) syndrome.4
Telaprevir and the other new DAAs are xenobiotics,5 that enter and accumulate inside cells where may cause various toxic effects as well as expected therapeutic effect. These molecules are detected by various intracellular sensors that operate primarily in the liver, where xenobiotic agents are inactivated by the cytochrome P450 system, and ultimately are excreted in the urine and in the faeces via biliary clearance.6 In some cases, these drugs may cause cytotoxicity and/or specific immune response if the cytochrome P450 detoxification system is not sufficient to prevent their potential noxious effects.7 Given the previous widespread use of telaprevir and the wellknown ACRs, that are related to its use, it would be of interest to the medical community to understand the mechanism of this drug adverse effect, given that it could happen with future antiviral drugs.
Case ReportA 50-year-old Caucasian female with histological documented HCV-related chronic hepatitis of grade 6, stage 2 according to Ishak’s score8 and HCV-RNA viral load of 3121951 IU/mL, had not responded to dual therapy. Therefore, she started a triple therapy including 180 µg/week of PEG-IFN α2a as well as a daily dose of RBV 1,000 mg and telaprevir 2,250 mg. To prevent a drug-induced rash, the patient was advised to avoid prolonged exposure to sun or other heat sources, as well as tight-fitting clothing. Moreover, the continuous use of a moisturizing cream was recommended.
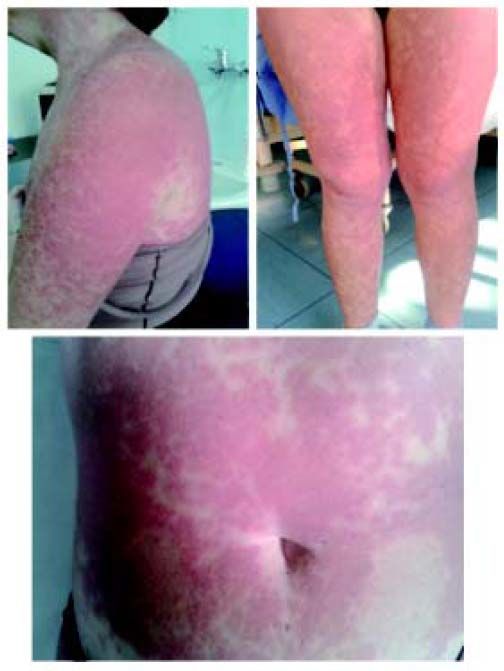
After four weeks HCV-RNA was detectable at 9,200 IU/mL, but after 2 more weeks, because of the decrease of haemoglobin levels below 10 g/dL, RBV was progressively reduced to 600 mg/day and recombinant erythropoietin was administered at dose of 40,000 IU/week. During the eighth week of treatment, eczematous and itchy lesion appeared on the patient’s chest and involved approximately 18% of body area, that is the grade 1 of telaprevir-associated rash severity.2 A treatment with topical steroids and oral antihistamines was ineffective so that cutaneous eruptions diffused on 36% of body area reaching the grade 2 of severity. As recommended,2 telaprevir was continued. However, the patient discontinued the entire treatment 2 days later for the rapid increase in severity of ACR to grade 3 with general malaise and fever up to 38.5 °C (Figure 1).
The patient was hospitalized at our unit because of the severity of the cutaneous reaction. The presence of leucocytosis, eosinophilia, moderate anaemia and the pre-existing liver dysfunction was compatible with a type of disease which could have evolved into DRESS syndrome, according to RegiSCAR criteria for diagnosis.9
A treatment with methylprednisolone 40 mg/die and clorphenamine maleate 20 mg/die by intravenous injection was started. The patient refused to undergo a skin biopsy. A report to the University Hospital Drug-Vigilance Service of adverse drug reaction was issued.
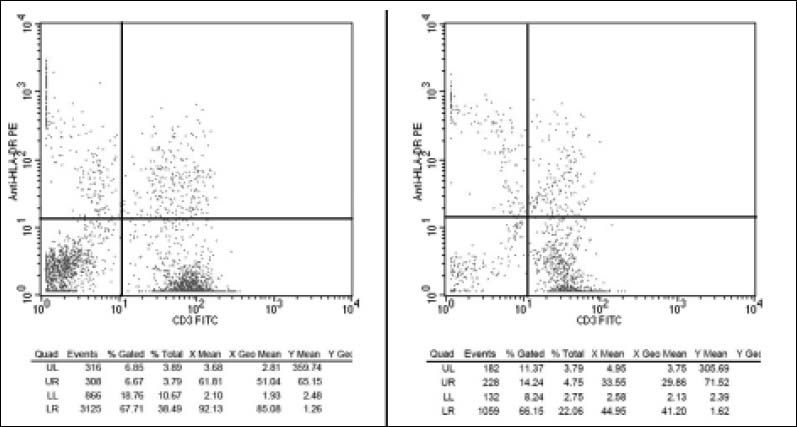
A lymphocytes transformation test10 was performed in the attempt of confirming telaprevir as the culprit drug for patient’s severe ACR and to evaluate its pathogenic mechanisms. Briefly, peripheral blood mononuclear cells (PBMCs) of the patient as well as three healthy subjects were isolated by density gradient and then cultured in triplicate for 38 h in medium containing 45 and 135 of telaprevir dissolved in Dimethyl-Sulphide. PB-MCs specimens cultured in pure medium or in medium containing 1 TSST-1 superantigen11,12 were respectively used as standard negative (basal) and positive control of T-cells activation. All PB-MCs cultures were incubated with a mix of fluorochrome-conjugated monoclonal antibodies and examined for their CD3, CD45 and HLA-DR expression13 by a flow cytometer. After multiple cytometric counts of 100 T-lymphocytes, the mean percentages of activated T-cells (CD3+/HDL-DR+ cells) were calculated. Activated T-cells were significantly higher in the patient than in healthy subjects (5.9 ± 0.8 vs. 1.19 ± 0.76; p < 0.05) in basal cultures. Also the increase of telaprevir activated T-cells was significantly higher in the patient than in control subjects (14.2 ± 4.2 vs. 1.52 ± 0.44; p < 0.05) (Figure 2). Finally, after stimulation with TSST-1, the increases of activated T-cells were similar both in the patient and in the controls.
Patient’s clinical features improved gradually over the next two days with a skull-caudal resolution of cutaneous eruptions and disappearance of fever. The complete healing of the lesions and the normalization of leucocytosis, eosinophilia and anaemia were obtained after one month. Six months later, in absence of any skin lesions and therapy, skin prick and intradermal testing were performed with telaprevir solutions of 0.047, 0.47 and 4.7 µg/mL,14 while skin patch testing was performed with telaprevir/vaseline mixtures (w/w) at 10%, 20% and 40%. Skin tests were performed on healthy subjects to evaluate any telaprevir skin irritating effect. The immediate and delayed responses were negative for all skin tests both in the patient and in healthy controls. After 6 months, the follow-up showed a persisting detection of HCV-RNA.
DiscussionTelaprevir, a DAA that inhibits the NS3-4A serine protease of HCV and suppresses HCV replication, was widely used in the recent past in order to treating chronic hepatitis C as a triple therapy including PEG-IFN and RBV. In trials with telaprevir-based triple therapy approximately half of patients reported ACRs and, importantly, a frequency of 6% of severe ACRs, such as SJS and DRESS syndrome, was observed.15 In case of these severe ACRs the treatment must be discontinued.16 In our case, entire treatment was discontinued for the rapid progression of ACR and the well-founded suspicion of DRESS syndrome, which was not subsequently confirmed.9,17
Until now, molecular mechanism underlying telaprevir induced ACRs as well as pathomechanisms of the DRESS syndrome are not defined, although the role of immune system is generally proposed.18–22 There are many reasons to suspect that an adverse reaction to a direct-acting antiviral, during a chronic active infection by HCV, may be induced by a T cell-dependent immune mechanism. During an active HCV infection, potential xenobiotic toxicity and antigenicity of direct-acting anti-HCV drugs5 may be increased because of altered liver systems of metabolism and detoxification,7 with overproduction of reactive and antigenic metabolites as the case of M11 metabolite of telaprevir.23 Drug metabolism is central in the starting, persistence and propagation of drug hypersensitivity because of drug metabolites that are generated in different quantities throughout the body. These metabolites can be directly or indirectly toxic to cells and stimulate innate immunity, as well as bind to protein to generate neoantigens for adaptive cellular and humoral responses.24 Most of drug metabolism occurs in the liver. However, liver-generated metabolites are unlikely to stimulate T-cell-mediated reactions in other organs, such as the skin, unless the metabolite avoids detoxification processes, and is stable enough to circulate.25 Because the skin or immune cells themselves express drug-metabolizing enzymes, these organs could be as important as the liver in bioactivating certain drugs, and could play a specific key role in drug-induced skin reactions and in directing the immune system towards a reaction rather than tolerance.26–28 In other words, some drugs are metabolized to reactive metabolites. This chemical reaction or detoxification of reactive metabolite, may generate some oxygen reactive species and decrease availability of the antioxidant pool, which can induce an oxidative stress for cells.29 Some drug metabolites are able to stimulate cells of innate and adaptive immune system and lead the immune system to actively react to the drug. The subsequent immune reactions, associated with drug metabolisminduce oxidative stress, could lead to tissue damage and explain clinical signs of drug hypersensitivity.24
Maculopapular exanthemas, bullous exanthemas, SJS and DRESS syndrome are all clinical delayed adverse reactions frequently observed during the drug treatment of hepatic or non hepatic viral infections. If they are expression of an immune-mediated hypersensitivity reaction to the used drug, it is likely that T-cells driven immune responses are involved.30 Moreover, during HCV infection as well as other viral infections, there are a lot of “danger signals”, such as the expression of costimulatory molecules by antigen presenting cells, oxidative stress or cell death, which would lead the immune system towards an active immune response.31
The clinical predictors of these adverse reactions are poorly understood. However, a recent study showed a significant association between telaprevirinduced dermatological reactions and elevated serum granulysin levels. Granulysin is a protein released by cytotoxic T lymphocytes and natural killer cells, that induces apoptosis in target cells and has antimicrobial activities. Serum granulysin levels were significantly associated with the severity of dermatological reactions. Therefore, the authors have supposed that this protein could be a useful predictor of telaprevir-induced dermatological reactions.32 Weston, et al. have described that telaprevir-associated cutaneous reaction activated inflammasomes in THP-1 cells, unlike the drug not associated with cutaneous reactions.33
In vitro, results demonstrated telaprevir induced lymphocyte activation in our patient. These lymphocyte activation strongly suggests that skin lesions are mediated by a drug-driven T cells activation. Otherwise, the relevance of lymphocyte activation in vitro is not affected by the negative results of the patch testing in vivo given the low sensitivity of this test in the delayed immune-mediated reactions induced by drugs.34,35 Moreover, skin tests were performed several months after ACR resolution, telaprevir discontinuance and the time of lymphocyte activation test. Considering the possibility of different biological scenarios at the different times of investigations, there are increased probability of divergent results between in vitro and in vivo test.
ConclusionIn conclusion, in the case studied, telaprevir induces adverse cutaneous reactions with a T-cell dependent immune mechanism. Therefore, in cases of suspected drug-induced severe reactions, like in our patient, an early lymphocyte transformation test may be a useful diagnostic aid.
Abbreviations- •
ACRs: adverse cutaneous reactions.
- •
DDAs: direct-acting antiviral agents.
- •
DRESS: drug reaction with eosinophilia and systemic syndrome.
- •
HCV: hepatitis C virus.
- •
PEG-IFN: peginterferon.
- •
RBV: ribavirin.