Cold liver preservation in the University of Wisconsin solution (UW) followed by reperfusion alters hepatic parenchyma and extra cellular matrix. In this study we analyzed the benefit of adding either 500 μM Sodium Nitroprusside (NPNa) or 100 μM S-nitrosoglutathione (GSNO) as Nitric Oxide (NO) donors to the UW solution to prevent hepatic injury. Wistar adult rat livers were stored in UW solution (0°C) for 48Hs and reperfused (60 minutes) in the isolated perfused rat liver model (IPRL). Untreated livers were used as normal controls. Livers perfused but not preserved were used as controls of reperfusion. Parenchyma damages were evaluated by Hematoxylin-Eosin stain. Picrosirius Red and Gordon-Sweets stains were used for collagen and reticulin networks, respectively. An inmunohistochemistry assay for albumin was used as functional test. Cold preservation step was followed by swollen hepatocytes with “light empty halos” surrounding the nucleus, conserved hepatocyte cords and many rounded endothelial cells. The addition of NPNa or GSNO into UW solution, avoid these alterations. Livers preserved for 48 Hs and then reperfused showed extended areas of vacuolation around central veins, and many endothelial cells were rounded and located inside sinusoidal lumens. The collagen network was disorganized while the reticulin one was less altered. Albumin was distributed preferentially in pericentral areas. On the contrary, livers preserved in presence of NPNa or GSNO did not show vacuolation and both collagen and reticulin networks were unchanged. Albumin was more homogeneously distributed in both groups. In conclusion, the addition of 500 μ NPNa or 100 μ GSNO as a NO donor, improves UW solution properties to preserve rat livers by maintaining the hepatic morphology and avoiding hepatic injury post-cold preservation/reperfusion.
Abbreviations:
UW: University of Wisconsin
NO: nitric oxide
NPNa: sodium nitroprusside
GSNO: S-nitrosoglutathione
KH: Krebs-Henseleit bicarbonate buffer
IPRL: isolated perfused rat liver
IntroductionThe University of Wisconsin solution (UW) has extended the scope and application of liver transplantation therapy.1 However, reperfusion of livers exposed to variable ischaemia time period during hepatic surgery can cause severe injury to hepatic tissue.2 Direct effects of cold ischaemia on livers produces cell swelling, interstitial edema, denudation of the sinusoidal lining cells by alterations in connections between cells and extra cellular matrix3,4 and activation of proteases.5,6 Extra cellular matrix damages cause changes on parenchymal and nonparenchymal cell morphology. Hepatic injury is further aggravated by disturbances of hepatic microvascular blood flow in the postischemic period that may be a consequence of nonparenchymal cell damage. These alterations increase vascular resistance, impeding the appropriate oxygen transport and delivery of nutrients from blood to hepatocytes. This phenomenon always precedes hepatocyte injury, suggesting that abnormalities in microcirculation could play a primary role in the pathogenesis of the graft nonfunction.7
Microcirculatory blood flow is modulated by vasoactive substances, such as Nitric Oxide (NO) and Endothelins.2 Alteration of intrahepatic concentrations of vasoconstrictors and vasodilators during reperfusion could therefore be responsible for postischemic impairment of hepatic blood flow.8
S-nitrosothiols are important NO donors, which exhibit endothelin, derived relaxing factor-like properties9 and have effects on vascular smooth muscle. S-nitrosoglutathione (GSNO) is a S-nitrosothiol that has proved to relax tracheal smooth muscle.10 Aside from its use as organic synthesis reagent, GSNO is of interest because its potent pharmacological properties and possible physiological role in smooth muscle relaxation like other NO donors such as Sodium Nitroprusside (NPNa).10,11 Meanwhile, NPNa is a potent vasodilator that releases NO by a non-enzymatic process and it acts as a direct vasodilator independent from endothelial function.12,13
In previous works we demonstrated that the addition of 100 μM GSNO or 500 μM NPNa to the UW solution, as NO donors, improved the morphology of rat livers cold preserved in UW solution during 48 Hs at 0°C and then reperfused using the Isolated Perfused Rat Model (IPRL).11,14
The purpose of this work was to compare the benefit of adding 100 μM GSNO or 500 μM NPNa to the UW solution and to determinate which of this two NO donors is more suitable to avoid preservation/reperfusion injuries on rat livers morphology when they are cold preserved in UW solution (48 Hs-0°C) and then reperfused using de IPRL model.
Materials and methodsAnimalsAdult male Wistar rats weighing 250-350 g were used for this study. Animals had free access to standard rat chow and tap water, and were not fasted before surgery. All operations were performed between 9 AM and noon. The experiments described in this report were conducted according to international regulations and approved by the National Council of Research in Argentina (CONICET).
SolutionsThe UW solution used in this study was modified11 based on the UW solution described by Beltzer.15 The composition of the modified UW solution was as follows: 100 mM Lactobionic Acid, 25 Mm KH2PO4, 5 mM MgSO4, 30 mM Raffinose, 5 mM Adenosine, 1 mM Al-lopurinol, 3 mM Glutathione (GSH), 0.25 mg/mL Streptomycin and 10 UI/mL Penicillin G. GSH was added to UW solution before use, UW solution was brought to pH 7.40 at 25°C with 5 M KOH. The final Na+ and K+ concentrations were 30 mM and 125 mM, respectively. The solution was bubbled with 100% N2 for 15 min at 0°C before use.16 Hydroxyethyl Starch, Dexametasone and Insulin were omitted with respect to the commercial solution, because their roles are still controversial.17 GSNO or NPNa was dissolved in a small volume of distillate water and added to UW solution immediately before use.
Composition of perfusate Krebs-Henseleit bicarbonate buffer (KH)–bovine serum albumin: the synthetic medium consisted in 2 % bovine serum albumin (Sigma A 4503) in KH with the following composition: NaCl 118 mM, KCl 4.8 mM, NaHCO3 25 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, CaCl2 1.5 mM, Heparin 2 UI/mL and Glucose 5 mM. The final pH of the KH solution after equilibration with carbogen (O2:CO2, 95:5 %) was 7.40. The perfusate was filtered through a 1.5 μm glass fiber filter before use.
Hepatectomy and cold storageThe rats were anesthetized with sodium tiopental (50 mg/ Kg, i.p.) and livers prepared according to the standard technique.18 In brief, the bile duct was cannulated with a PE-50 catheter (Intramedic USA) and 0.2 mL of saline containing 500 UI of Heparin (Abbot, Argentina) was injected into the femoral vein. The portal vein was cannulated with a large catheter 14 G (2.10 mm internal diameter) and the hepatic artery were ligated. The liver was perfused with oxygenated KH buffer (37°C) at a flow of 15 mL/min. The suprahepatic inferior vena cava was then cannulated with a polyethylene tubing (3 mm internal diameter) and the liver was removed without stopping the perfusion. In the control group the perfusion was performed immediately, whereas in the experimental groups the livers were flushed-out with 30 ml of cold UW solution for approximately 2 min and then transferred to a vessel containing the preservation solution maintained at 4°C. At the end of this procedure, the livers were cold stored at 0°C for 48 hours.
ChemicalsGSNO was prepared according to the method of Hart19 by incubating equimolar amounts of glutathione and sodium nitrite in acidified water at 0°C. GSNO solutions were freshly prepared before each experiment from GSNO powder conserved at 4°C under vacuum. Preparation quality and concentration of GSNO was spectrophotometrically determined at 335 nm (E ϵ826 dm3 mol-1 cm-1) and at 545 nm (E ϵ17.4 dm3 mol-1 cm-1). NPNa was purchased from Merck (AG, Germany).
The perfusion systemThe livers were perfused using a recirculating system modified from Alexander et al.20 as we described previously.11,21,22
Histological studiesTo evaluate histological changes produced during cold preservation/reperfusion, pieces of livers were taken from each experimental group (n = 5). Tissues were fixed in 4% PBS buffered formalin (pH = 7.40) and embedded in paraffin. Sections were cut at 5 μm thick and processed as follows:
- •
Hematoxilin-eosin stain was performed to evaluate hepatocyte cords integrity; presence of vacuoles, blebs and necrotic focus; sinusoidal endothelial cells shape; and the morphological aspect of the hepatic lobules.
- •
Picrosirius red stain was used to analyze the collagen network.23
- •
Gordon-Sweet stain was used to analyze the reticulin network.24
- •
Immunohistochemistry for albumin was done to study the content of hepatocyte albumin after cold preservation/ reperfusion as a parameter of viability for parenchymal cells. Immunohistochemistry was performed using an antibody against albumin (Serotec-UK) as we described previously.14 Briefly, paraffin sections were prepared and microwaved for antigen recover (800 W, 4'30", temperature lower than 85°C) in citrate buffer pH = 6.00, and cooled under tap water. Liver sections were then incubated with anti-albumin (diluted 1:2,000) after nonspecific binding was blocked with 5% non-fat milk in buffer TBS, for 40 min in hummed chamber at room temperature. After incubation with ABC system (Vector Laboratories, USA), sections were incubated with Fast Red solution at 37°C for 25 minutes according to manufacture directions, counterstained with Hematoxylin and mounted with glycerol buffered.
- I)
normal controls consisted in livers neither preserved nor reperfused (IC);
- II)
reperfusion controls, in which hepatectomy was immediately followed by the organ perfusion in the IPRL model during 60 min (IIRC)
To study the effect of GSNO and NPNa added to UW solution on liver morphology during cold preservation we analyzed the following groups:
- III)
Livers cold preserved during 48 Hs at 0ºC in UW solution (IIIP48);
- IV)
Livers cold preserved during 48 Hs at 0ºC in UW solution with the addition of 500 mM NPNa (IVPNPNa);
- V)
Livers cold preserved during 48 Hs at 0ºC in UW solution with the addition of 100 μM GSNO (VPGSNO);
To investigate the effect of cold preservation following by reperfusion we analyzed the following groups:
- VI)
Livers cold preserved (0ºC-48 Hs) in UW solution and then reperfused (VIP/R48);
- VII)
Livers cold preserved (0ºC-48 s) in UW solution with 500 μM NPNa and then reperfused (VIIP/RNPNa);
- VIII)
Livers cold preserved (0°C-48 Hs) in UW solution with 100 μM GSNO and then reperfused (VIIIP/RGSNO).
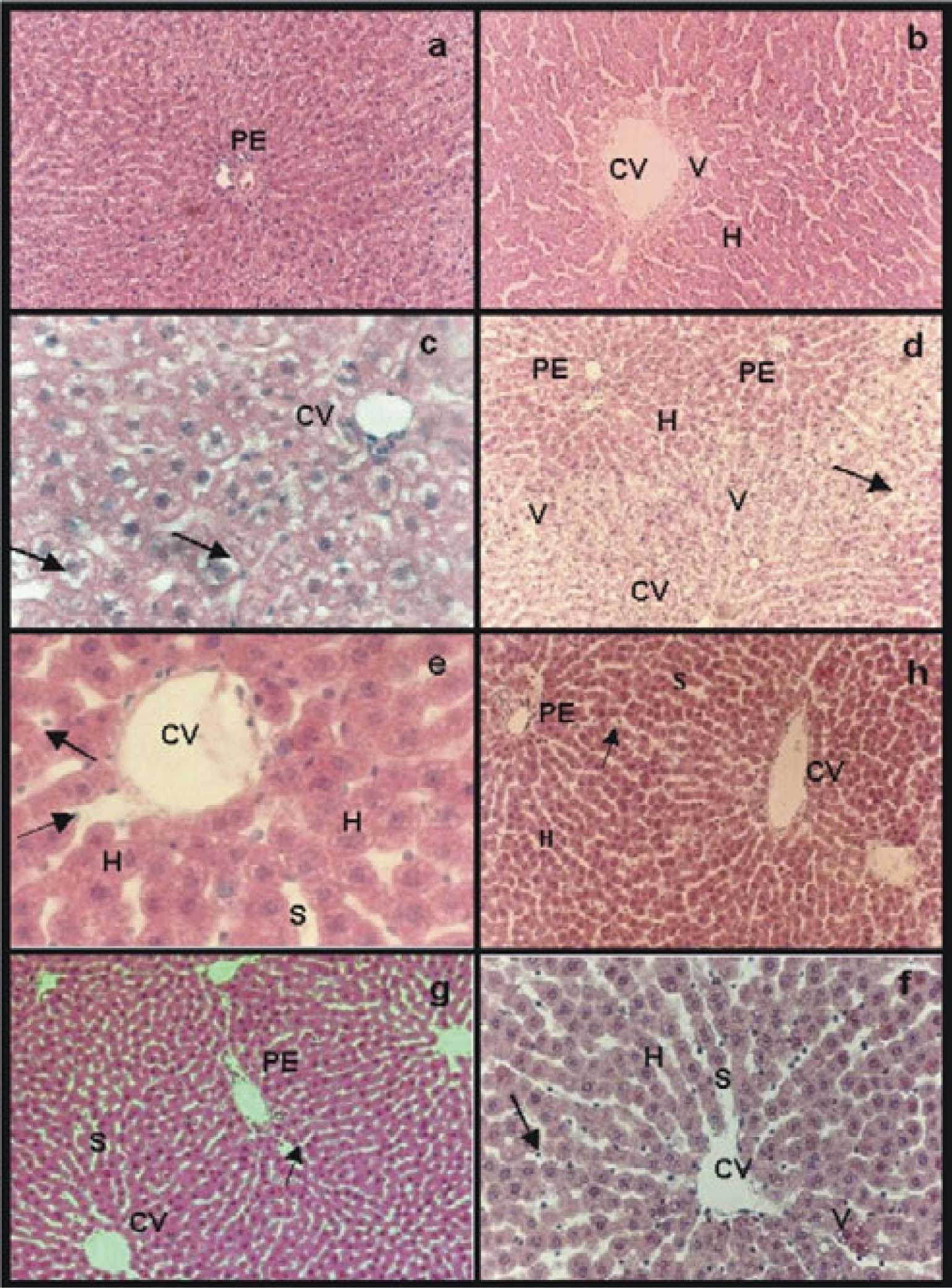
Group IC was used to compare the histology of normal livers with the hepatic morphology of treated groups (preserved and preserved/reperfused) (Figure 1a). In group IIRC we observed the presence of few vacuoles around central veins, sinusoid lumens appeared wider than in group IC and some sinusoidal endothelial cells showed rounded shape, hepatocytes cords were not disorganized and no necrotic focus or great damages were seen within the hepatic lobule. Morphological alterations of this group represented the damages caused on hepatic structure by the IPRL system itself (Figure 1b).
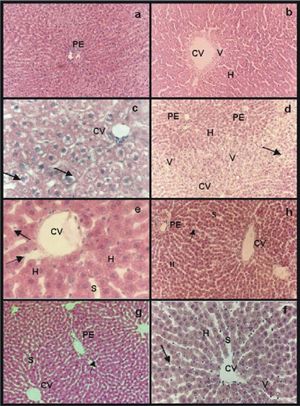
Hematoxilin-eosin stain: a) group IC, normal parenchyma around a portal space (PE); b) group IIRC, small area of vacuolation (V) around central vein (CV). Sinusoids appeared wider than in normal controls (S); c) group IIIP48, swollen hepatocytes with “light empty halos” surrounding the nucleus (arrows) and sinusoids with a grater caliber than the ones of group IC (S); e) IVpNpNa and g) group VpGSNO, in both groups “light empty halos” disappeared while sinusoids presented wider caliber than controls (S), swollen hepatocytes (H) and some rounded endothelial cells were seen (arrows); d) group VIP/R48, extended areas of vacuolation (V) around central veins (CV) with portal areas (PE) without vacuoles. Hepatocytes were swollen (H). Sinusoidal endothelial cells were all rounded and most of them appeared inside sinusoidal lumen (arrow); f) group VIIP/RNPNa and h) group VIIIP/RGSNO, less endothelial cells were inside sinusoidal lumen (arrows), sinusoid were dilated (S). The only difference between group VIIP/RNPNaand group VIIIP/RGSNO (Figure lh)was that the last group presented few vacuoles (V) around central veins with normal portal areas. Magnifications: a, b, d, h, g: 41x, f: 83x and e, c: 165x.
The morphology of group IIIP48 showed swollen hepatocytes with “light empty halos” surrounding the nucleus but the hepatocyte cords were conserved. Sinusoids had a grater diameter than the ones of group IC and many rounded sinusoidal endothelial cells were seen (Figure 1c).
In group IVPNPNa alterations seen in group IIIP48 were avoided significantly. While the “light empty halos” disappeared, the other alteration were present (Figure 1e). The same results were obtained for group VPGSNO(Figure 1g).
Reperfused groups:Group VIP/R48 showed extended areas of vacuolation around central veins with portal areas without vacuoles. Hepatocytes were swollen but hepatocyte cords integrity was preserved. Sinusoidal endothelial cells were all rounded and most of them appeared inside sinusoidal lumen (Figure 1d).
In group VIIP/RNPNa not vacuolation was seen, and less endothelial cells were inside sinusoidal lumen. Nevertheless, most endothelial cells appeared rounded and sinusoid were dilated. The parenchyma was organized and most conserved than in group VIP/R48(Figure 1f).
The only difference between group VIIP/RNPNa and group VIIIP/RGSN O (Figure 1h) was that the last group presented few vacuoles around central veins with normal portal areas.
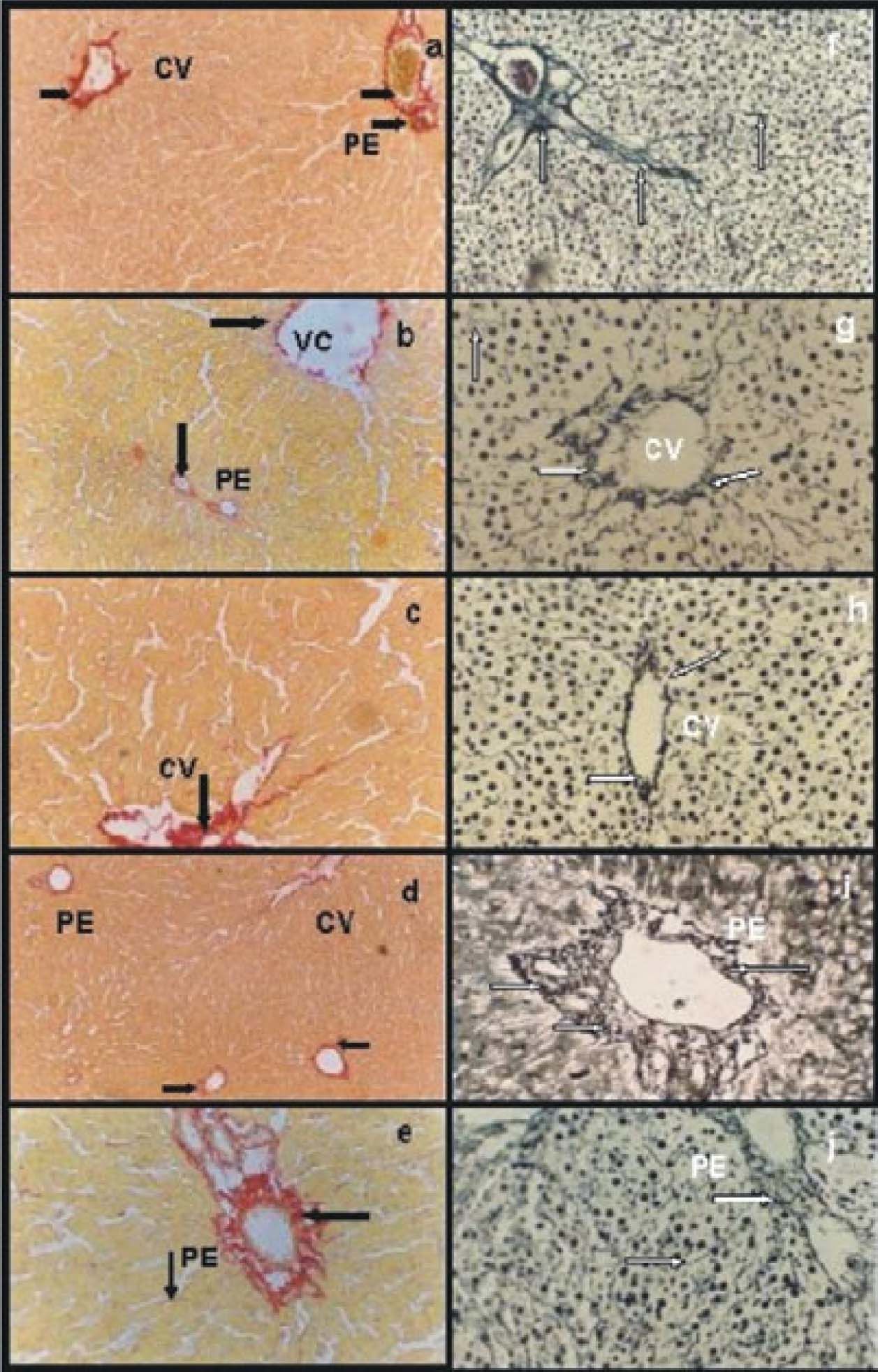
Picrosirius red and Gordon-Sweet stainsControlsGroup IC showed networks of collagen (Figure 2a) and reticulin (Figure 2f organized with abundant fibers. Group IIRC presented certain disorganization of both collagen (Figure 2b) and reticulin (Figure 2g) networks an apparently with less fibers compared with group IC.
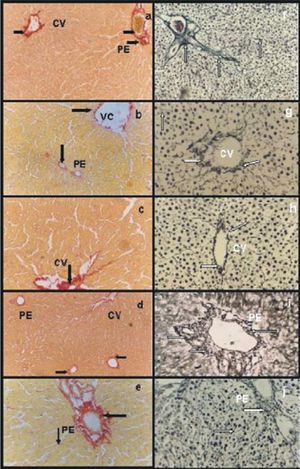
Picrosirius red stain: a) group IC, abundant and organized network of collagen type I and III around a portal space (PE) and central vein (CV) (arrows); b) group IIRC, certain disorganization of both collagen network and apparently with less fibers compared with group IC (arrows); c) group VIP/R48, disorganized networks of collagen with less fibers than group IC (arrow); d) group VIIP/RNPNa and e) group VPGSNO Groups VIIP/RNPNa, both groups had organized networks of collagen and the amount of fibers were similar to group IC. Gordon-Sweet stain: f) group IC abundant and organized network of reticulin around a portal space (PE) and within the entire parenchyma (arrows); g) group IIRC, Group IIRC certain disorganization of reticulin network and apparently with less fibers compared with group IC (arrows); h) group VIP/R48, reticulin network was more organized around central veins (CV) and portal areas, but the amount of fibers seemed to be diminished compared with IC (arrows); i) group VIIP/RNPNa and j) group VIIIP/RGSNO, both groups presented an organized networks reticulin around portal areas (PE) and central veins, and the amount of fibers were similar to group IC (arrows). Magnifications: a, b, d, f, j: 41x; c, e, h, i: 83x.
Groups IIIP48 5PNPNa and VPGSNO M n° relevant differences of collagen and reticulin networks compared with group IC (data not shown).
Reperfused groupsGroup VIP/R48 presented disorganized networks of collagen with fewer fibers than group IC(Figure 2c). Reticulin network was more organized but the amount of fibers seemed to be diminished compared with IC(Figure 2h).
Groups VIIp/RNPNa had organized networks of collagen (Figure 2d) and reticulin (Figure 2i) and the amount of fibers were similar to group IC. The same results were obtained for group VIIIp/RGSNO.(Figure 2e, collagen; Figure. 2j, reticulin).
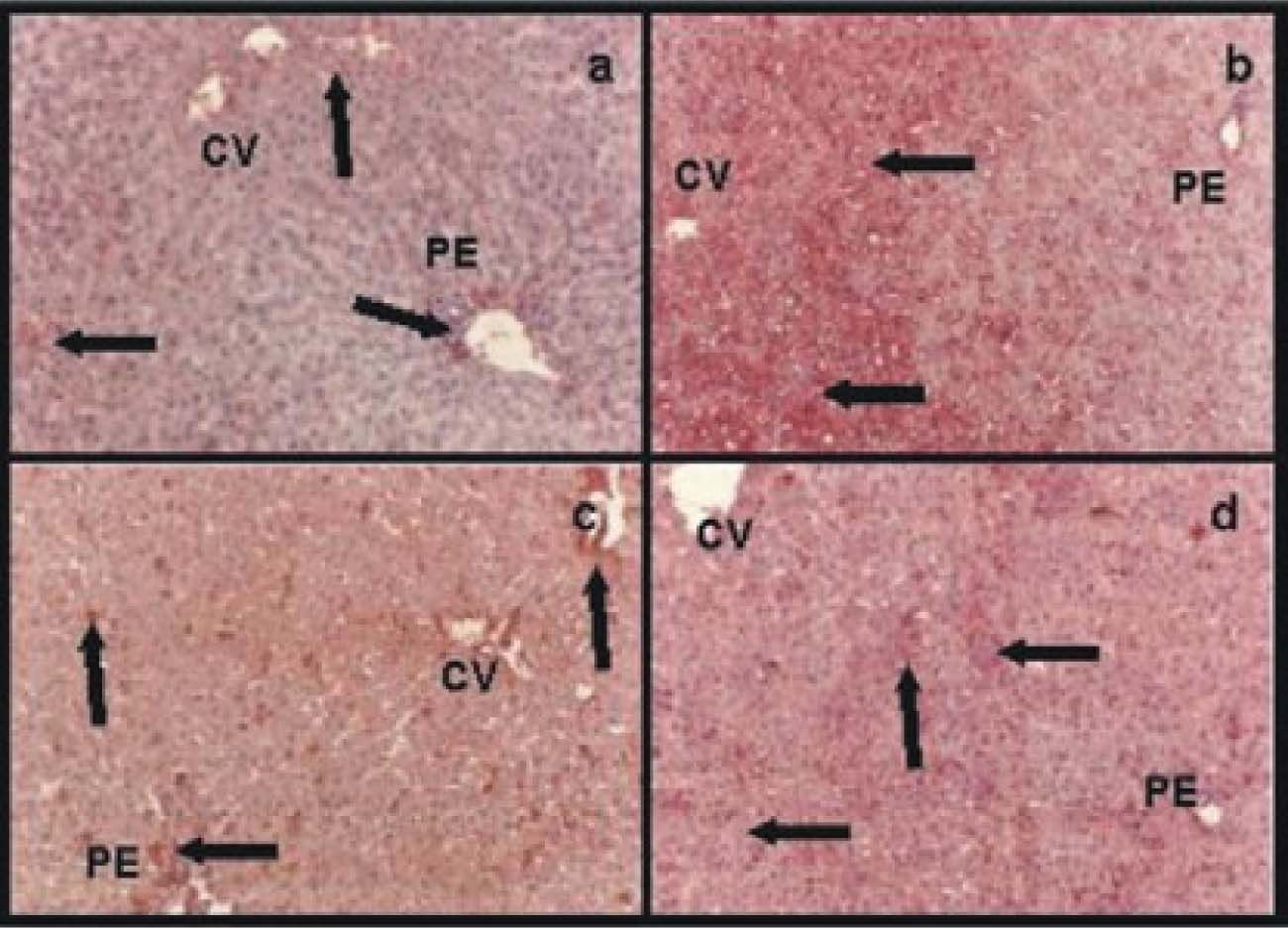
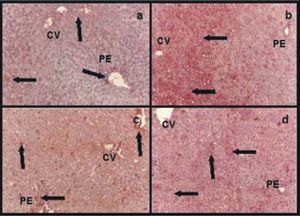
Immunohistochemistry for albuminControlsIn group IC albumin appeared homogeneously distributed within the hepatic lobules (data not shown). Group IIRC showed less amount of albumin, which was more abundant around central veins and portal areas (Figure 3a).
Immunohistochemistry for albumin: a) group IIRC, less amount of albumin than in normal controls which was more abundant around central veins (CV) and portal areas (PE); b) group VIP/R48, an heterogeneous distribution of albumin within the hepatic lobule. The great amount of albumin was present in pericentral areas (CV) and the less amount in periportal ones (PE) (arrows); c) group VIIP/RNPNa and d) group VP/RGSNO, both groups had no differences for albumin distribution within the hepatic parenchyma. Albumin was more homogeneous distributed than in group VIP/R48 (arrows). Magnification: 41x.
IVPNPNa and VPGSNO had no differences compared with group IC (data not shown).
Preserved groupsGroup VIP/R48 showed a heterogeneous distribution of albumin within the hepatic lobule. The great amount of albumin was present in pericentral areas and de less amount in periportal ones (Figure 3b).
Groups VIIP/RNPNa(Figure 3c) and VIIIP/RGSNO(Figure 3d) had no differences for albumin distribution within the hepatic parenchyma. Albumin was more homogeneous distributed than in group VIP/R48.
DiscussionIschemia causes morphological alterations on livers cold preserved in UW solution (48 Hs – 0°C). Reperfusion with the IPRL model after cold preservation aggravates theses damages.11,14 Cold preservation of rat livers in UW solution for 24 Hs is not a severe condition leading to primary liver non-function. Longer periods of cold preservation should be used when studying the hepatophysiologic mechanisms involved in the cold-ischemia injury of rat liver.25 In previous studies we hence demonstrate that rat livers stored in UW solution up to 48 Hs shown severe morphological and hemodynamics alterations indicating that 48 Hs is the timeline to preserve rat livers under hypoxia and hypothermia.11,14,21,22 However, these alterations could be reversed by adding 500 μM NPNa11 or 100 μM GSNO.14,22
Morphological alterations of parenchymal and non-parenchymal cells (hepatocytes and endothelial cells) seen in group IIIP48 represented long term of hypotermic preservation (48 Hs) under hypoxia. Swollen hepatocytes and disruption of the endothelial cell line are described as ones of the primary injuries seen during cold preservation of livers in UW solution.3,4 These alterations were observed in group IIIP48 and in addition, perinuclear “light empty halos” were also seen in all hepatocytes within the hepatic lobule. This halos were not present when 500 μM NPNa or 100 μM GSNO was added to UW solution. This phenomenon might represent reversible alterations characteristic of cold preservation and hypoxia.
Groups IVPNPNa and VPGSNO improved liver morphology, diminishing the alterations seen in group IIIP48 The improvement of UW solution with the addition of NO donors in an adequate concentration had been proved previously.11,14 In this study we demonstrate that the addition of 500 μM NPNa or 100 μM GSNO avoided parenchymal and nonparenchymal cells morphology damages during cold preservation under our experimental conditions (48 hs-0°); no differences between both NO donor in preventing preservation/reperfusion injuries could be established with our results.
Collagen and reticulin networks were not significantly altered in preserved groups. This finding might be due to the fact that during cold preservation, proteases are activated5,6 and the damages produced by this phenomenon are clearly manifested after reperfusion.
After long terms of hypoxia, reoxygenation during reperfusion causes an increment of superoxide anion concentration, especially in middle zones of the hepatic lobule, producing superoxide-mediated cytotoxicity.26,27 This could explain the extended vacuolated areas seen around central veins and middle zones, with normal portal areas, observed in group VIP/R48. Livers of this group showed more albumin distribution around perivenous hepatocytes rather than in periportal areas. Since perivenous hepatocytes were the ones that showed their cytoplasm full of vacuoles, it could be assumed that these cells were damaged and could be the first to lose viability. Nevertheless, the content of albumin was higher than in hepatocytes of portal areas were no vacuolation was seen. These results allow us to conclude that albumin secretion is not appropriate to estimate hepatocyte viability after cold preservation/reperfusion period. Collagen and reticulin networks were disorganized comparing with group IC. This could be related to protease activation.5,6 Matrix metalloproinases, particularly metalloproteinases 2 and 9, are activated during cold preservation.6 Since collagen type I is one of matrix metalloproteinase 2 substrate,28 it seems possible that the activation of these metalloproteinase during cold preservation induced collagen and reticulin network disorganization and sinusoidal endothelial cell detachment which is more evident after reperfusion.
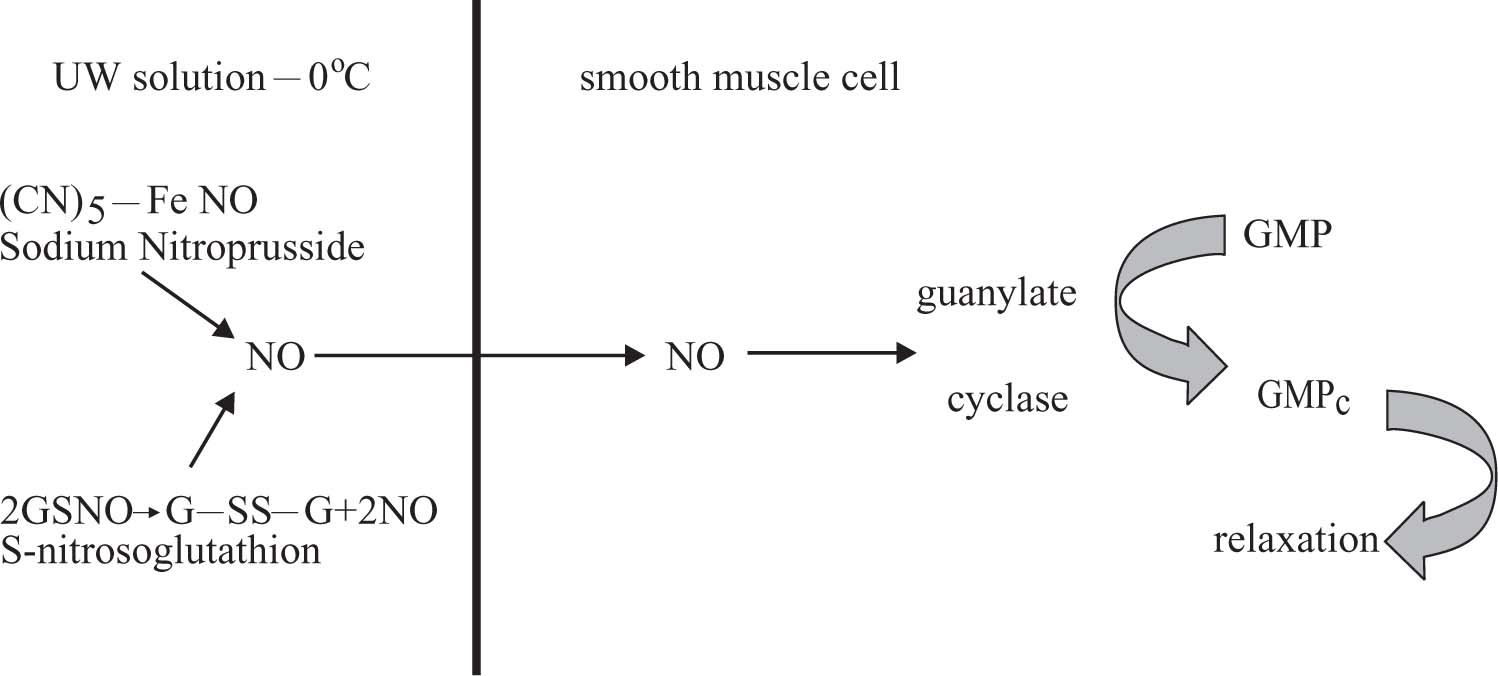
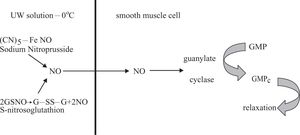
During cold preservation there is an increment of endothelins due to hypoxia and this phenomenon produces vasoconstriction. In addition, the high concentration of potassium in UW solution aggravates this phenomenon.11 To improve hepatic hemodynamic and morphology during cold preservation in UW solution (0°C-48 Hs) following by reperfusion, GSNO or NPNa was added.11,14,22 These compounds can generate NO, which is vasodilator that produces relaxation on smooth muscle cells according to the reactions shown in figure 4.
NPNa donors NO to UW solution by a nonezymatic process;12,13 meanwhile GSNO is oxidized to G-SS-G releasing NO to UW solution.29 NO can diffuse though cells citoplasmatic membranes easily. NO generated in UW solution from NPNa or GSNO enters the smooth muscle cells and activates guanylate cyclase to transform GMP to GMPC. The last compound produces relaxation.30
The addition of NPNa or GSNO reversed many morphological alterations seen in group VIP/R48. There were no significant differences among the results obtained with both NO donors and we assumed that either NPNa or GSNO is suitable as a UW solution component to avoid cold preservation/reperfusion injuries. Nonetheless, GSNO has a physiological mechanism to generate and storage NO in cells. S-nitrosothioles are present in cells and they can promote relaxation naturally. Accordingly, we suggest that GSNO could be a better additive for UW solution to prevent hepatic morphological alteration during cold preservation/reperfusion rather than NPNa.
AcknowledgmentsThe authors acknowledge Histotechnologist Alejandra Inés Martinez for her excellent technical assistance. This study was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) of República Argentina. PICT - 05-06434, BID 1201 OC/AR