Introduction. The application of nucleos(t)ide analogues in hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACLF) has not yet been widely accepted. Therefore, we conducted a meta-analysis of prospective and retrospective studies to examine the efficacy and safety of nucleos(t)ide analogues in treating HBV-related ACLF.
Material and methods. Two independent reviewers identified eligible studies through electronic, and manual searches, and contact with experts. Three-month mortality was defined as the primary efficacy measure. ACLF reactivation and HBV DNA inhibition were secondary efficacy measures. Quantitative meta-analyses were performed to compare differences between nucleos(t)ide analogue and control groups.
Results. Five eligible studies were identified. Antiviral treatment with nucleos(t)ide analogues led to significant reduction of HBV DNA [HBV DNA reduction > 2 log: 70.4 vs. 29%, RR = 2.29, 95%CI (1.49, 3.53), P < 0.01]. ACLF patients receiving nucleos(t)ide analogue had significantly lower 3-month mortality [44.8 vs. 73.3%, RR = 0.68, 95%CI (0.54, 0.84), P < 0.01] as well as incidence of reactivation [1.80 vs. 18.4%, RR = 0.11, 95%CI (0.03, 0.43), P < 0.01] compared to those who did not. There was no significant difference in the prognosis of patients treated with entecavir or lamivudine [36.4 vs. 40.5%, RR = 0.77, 95%CI (0.45, 1.32), P = 0.35]. No drug-related adverse events were reported during follow-up.
Conclusion. Our findings suggest that nucleos(t)ide analogue treatment reduces short-term mortality as well as reactivation of HBV-related ACLF patients. Nucleos(t)ide analogues are well-tolerated during therapy, and suggestive evidence indicates that entecavir and lamivudine confer comparable short-term benefits in these patients. However, further studies are needed to clarify these observations.
Chronic hepatitis B virus (HBV) infection is a major global health burden with approximately 350 million people chronically infected.1 It is well-known that individuals with chronic HBV infection are at increased risk of developing long-term complications of cirrhosis including hepatic de-compensation and hepatocellular carcinoma (HCC).1,2 In some cases, patients with chronic hepatitis B (CHB) may develop severe acute exacerbations, resulting in liver failure and death.3 This clinical entity, sometimes named as ‘severe flare-up of chronic hepatitis B’4,5 or ‘chronic severe hepatitis B’,6 was recently defined as HBV-related acute-on-chronic liver failure (ACLF).3 HBV-as-sociated ACLF has been associated with extremely high short-term mortality ranging from 30-70% according to several reports.4,5,7 Liver transplantation is the only definitive treatment for ACLF patients who did not respond to supportive measures. However, liver transplantation is limited by the availability of donor organs and high medical expense.
The efficacy of nucleos(t)ide analogues has been confirmed for the treatment of CHB.1,2 However, whether antiviral therapy would be effective for HBV-related ACLF remains controversial. There have been several studies on the use of nucleos(t)ide analogues in these patients.4,7–10 However, the results have been conflicting, and most limited by small sample sizes, and different criteria for patient enrollment. The aim of the current study was to allow data combination among different studies, using the ACLF criterion proposed by the Asian Pacific Association for the Study of the Liver (APSAL)11 to examine the efficacy and safety of nucleos(t)ide analogues for the treatment of HBV-related ACLF.
Material and MethodsSearch strategy and eligibility criteriaTwo independent investigators searched Pubmed, Embase, Chinese Medical Database (CBM), Chinese National Knowledge Infrastructure (CNKI), Wanfang data and VIP information for eligible articles up to Dec. 10, 2011. We applied a free key word or mesh word searching with the following terms: nucleos(t)ide analogues, lamivudine (LAM), tenofovir (TDF), entecavir (ENT), adefovir (ADV), telbivudine (LDT), acute-on-chronic liver failure, chronic severe hepatitis B, severe flare-up chronic hepatitis B, severe acute exacerbation chronic hepatitis B, and hepatic failure with hepatitis B. In addition, we also reviewed the bibliographies of relevant articles for articles not found by database searches. Disagreements were resolved by discussion and consensus between the two reviewers.
We included any randomized controlled trials that compared the mortality of HBV-related ACLF patients between antiviral treatment and control groups. We also included observational cohort studies if they provided data for calculation of the risk estimates of mortality in relation to antiviral therapy. Additionally, ACLF patients in eligible studies were required to have been diagnosed based on or within APASL criterion (Table 1). We excluded studies without clear declaration of study designs, studies lacking control groups, not published as full text or not published in English or Chinese. Additionally, if two or more studies were derived from overlapping populations, we only adopted the larger one.
Selection criteria of included trials.
| Study and year | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Garg 2010 | 1. ALT > 5 NL. | 1. Super-infection with hepatitis E, A, C or D or co-infection with HIV. |
| 2. HBV DNA > 105copies/mL. | 2. Other causes of chronic liver failure. | |
| 3. INR > 1.5. | 3. Coexistent HCC, portal vein thrombosis, renal impairment, pregnancy. | |
| 4. Total bilirubin ≥ 5 mg/dL. | ||
| 5. Presence of ascites and/or encephalopathy within 4 weeks. | 5. Previous antiviral, immuno-modulator or cytotoxic/immunosuppressive therapy within 12 months. | |
| 6. Presence of chronic liver diseases. | ||
| Hu 2010 | 1. 16 < age < 65. | 1. Super-infection with other hepatitis virus. |
| 2. Presence of serum HBV DNA. | 2. Coexistent with cancers, pregnancy or any other significant disease which might have interfered with the conduct of the study. | |
| 3. PTA ≤ 40%. | ||
| 4. Total bilirubin ≥ 10 mg/dL ora daily increase ≥ 1 mg/dL. | ||
| 5. Recent development of liver failure-related complications. | ||
| Chen 2011 | 1. 18 < age < 65. | 1. Co-infection of hepatitis A, C, D, E, Epstein-Barr virus, or other virus infections. |
| 2. HBV DNA > 3 log10 IU/mL. | 2. Other causes of chronic liver diseases such as drug-induced hepatitis, Wilson’s disease, alcoholic liver disease and autoimmune hepatitis. | |
| 3. Total bilirubin ≥ 5 mg/dL. | ||
| 4. PTA ≤ 40% or INR > 1.5. | ||
| 5. Recent development of complications such as ascites, HE,SBP, or HRS. | ||
| Cui 2010 | 1. 18 < age < 65. | 1. Co-infection of hepatitis A, C, D, E, or HIV. |
| 2. HBsAg(+) for at least 6 months duration. | 2. Other causes of jaundice. | |
| 3. HBV DNA > 103 copies/mL. | 3. Other causes of prolonged prothrombin time. | |
| 4. Total bilirubin ≥ 5 mg/dL. | 4. Other causes of chronic liver diseases. | |
| 5. PTA £40% or INR > 1.5. | 5. The presence of comorbid condition, uncontrolled metabolic condition or psychiastric condition. | |
| 6. Recent development of complications such as ascites, HE, SBP or HRS. | ||
| Sun 2009 | 1. The presence of HBsAg(+) for at least 6 months. | 1. Co-infection of hepatitis A, C, D, E, Epstein-Barr virus, cytomegalovirus or HIV. |
| 2. Serum HBV DNA > 104 copies/mL. | 2. Previous antiviral, immuno-modulator or cytotoxic/immunosuppressive therapy within 12 months. | |
| 3. ALT > 5 NL. | 3. Presence of hepatic decompensation before enrollment. | |
| 4. Total bilirubin > 5 mg/dL. | 4. Suspicious HCC. | |
| 5. PTA ≤ 40%or INR > 1.5. | 5. Other causes of chronic liver diseases. | |
| 6. Complicated with ascites and/or encephalopathy within 4 weeks; | 6. Other causes of jaundice and other causes of prolonged prothrombin time. |
HBV: hepatitis B virus. HBsAg(+): hepatitis B surface antigen positive. ALT: alanine aminotransferase. INR: international normalized ratio. HIV: human immunodeficiency virus. HCC: hepatocellular carcinoma. PTA: prothrombin activity. HE: hepatic encephalopathy. SBP: spontaneous bacterial peritonitis. HRS: hepatorenal syndrome. NL: upper limit of normal.
Two investigators independently extracted the following information for each study: study design, year, country or area, number and characteristics of study participants, pattern of nucleos(t)ide analogues drugs, dose, length of follow-up, end-points and risk estimates or relevant data able to calculate them. Any disagreement was resolved by consensus between reviewers.
Short-term mortality was the primary efficacy measure for this analysis. Recurrence, safety and virological response were used as secondary endpoints. Short-term mortality was defined as death due to any cause at 3 months from admission to hospital. Reactivation was defined as re-appearance of HBV-related ACLF in surviving patients during follow-up. Virological response was defined as HBV DNA reduction more than 2 log10 (IU/mL or copies/ mL). Adverse events were defined as any abnormal symptoms related to any nucleos(t)ide analogues during follow-up.
Quality assessmentFor randomized controlled trials, the methodological quality was evaluated by the Jadad scale,12 which examined randomization, blinding and reporting or subject withdrawal and dropout. Studies with scores ≥ 4 were considered as high-quality.
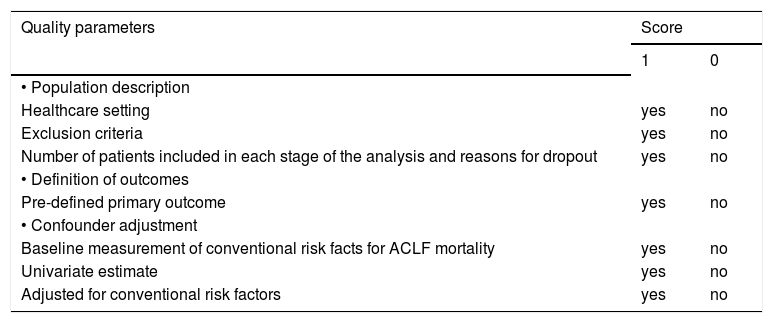
For observational cohort studies, the methodological quality was assessed using a 7-point scoring system measured by population description, definition of outcomes and confounder adjustment (Table 2). Studies with an overall score ≥ 5 were classified as high-quality. This scoring system was simplified from the model proposed by Hemingway, et al.13
Quality criteria of retrospective cohort studies included in the meta-analysis.
| Quality parameters | Score | |
|---|---|---|
| 1 | 0 | |
| • Population description | ||
| Healthcare setting | yes | no |
| Exclusion criteria | yes | no |
| Number of patients included in each stage of the analysis and reasons for dropout | yes | no |
| • Definition of outcomes | ||
| Pre-defined primary outcome | yes | no |
| • Confounder adjustment | ||
| Baseline measurement of conventional risk facts for ACLF mortality | yes | no |
| Univariate estimate | yes | no |
| Adjusted for conventional risk factors | yes | no |
We used relative risks (RR), and the corresponding 95% confidence intervals as effect measurements. All unadjusted RRs were calculated using available data. To combine crude risk estimates, quantitative meta-analysis was performed using Revman version 5.1 (The Nordic Cochrane Center, The Cochrane Collaboration). Both the Cochrane’s Q test and I2 measurement were conducted to evaluate intra-study heterogeneity. Substantial heterogeneity was indicated if P value was ≤ 0.10 or I2 was ≥ 50%. However, we used a random effect model irrespective of presence of significant heterogeneity to allow comparisons among different pooled risk estimates. Publication bias was evaluated by funnel plots and Egger test.14 An asymmetry plotting or P value ≤ 0.10 indicated presence of publication bias. Sensitivity analysis was performed to evaluate the validity and reliability of primary meta-analysis.
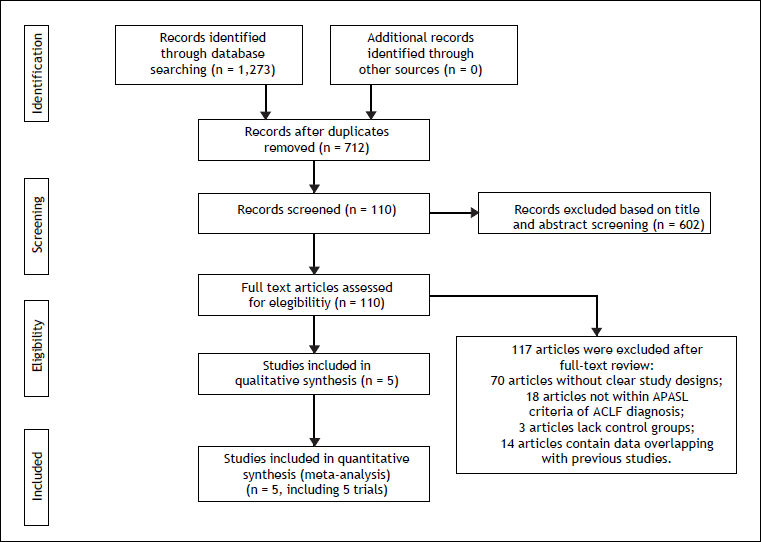
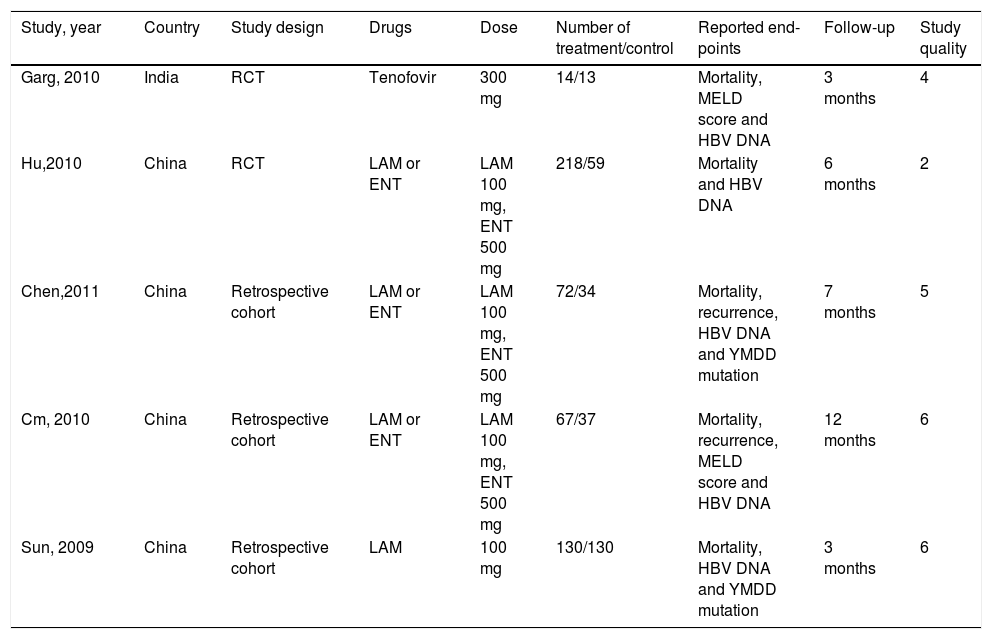
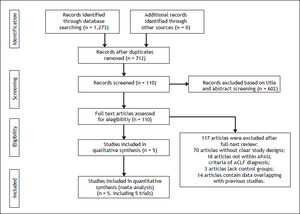
ResultsSearch results and characteristics of included studiesOf the 1,273 references identified, 562 duplicates were deleted. Information including title, abstract and full-text screening obtained 5 studies involving 765 patients (Figure 1).15–19 All the ACLF patients included in the studies were met APASL diagnostic criterion. Among them, 215,16 were RCTs and the remainder 17-19 were retrospective cohort studies. All the studies were conducted in China and one in India.15 Two studies15,19 exclusively used one nucleos(t)ide analogue (one with LAM and one with tenofovir), while 316–18 used two drugs (LAM and ENT). The doses of antiviral drugs were uniform in the included studies. Patients in 2 studies15,19 were followed for 3 months, while the remainder16-18 had a longer follow-up. According to the Jadad scale and the scoring system developed by us, 4 studies15,17–19 were of high methodological quality and one16 was not. All the articles were published in English except one in Chinese. All studies were published as full-text articles (Table 3).
Characteristics of included studies.
| Study, year | Country | Study design | Drugs | Dose | Number of treatment/control | Reported end-points | Follow-up | Study quality |
|---|---|---|---|---|---|---|---|---|
| Garg, 2010 | India | RCT | Tenofovir | 300 mg | 14/13 | Mortality, MELD score and HBV DNA | 3 months | 4 |
| Hu,2010 | China | RCT | LAM or ENT | LAM 100 mg, ENT 500 mg | 218/59 | Mortality and HBV DNA | 6 months | 2 |
| Chen,2011 | China | Retrospective cohort | LAM or ENT | LAM 100 mg, ENT 500 mg | 72/34 | Mortality, recurrence, HBV DNA and YMDD mutation | 7 months | 5 |
| Cm, 2010 | China | Retrospective cohort | LAM or ENT | LAM 100 mg, ENT 500 mg | 67/37 | Mortality, recurrence, MELD score and HBV DNA | 12 months | 6 |
| Sun, 2009 | China | Retrospective cohort | LAM | 100 mg | 130/130 | Mortality, HBV DNA and YMDD mutation | 3 months | 6 |
ACLF: acute-on-chronic liver failure. RCT: randomized controlled trial. LAM: lamivudine. ENT: entecavir. LDT: telbivudine. ADV: adefovir. MELD: the model for end-stage liver disease.
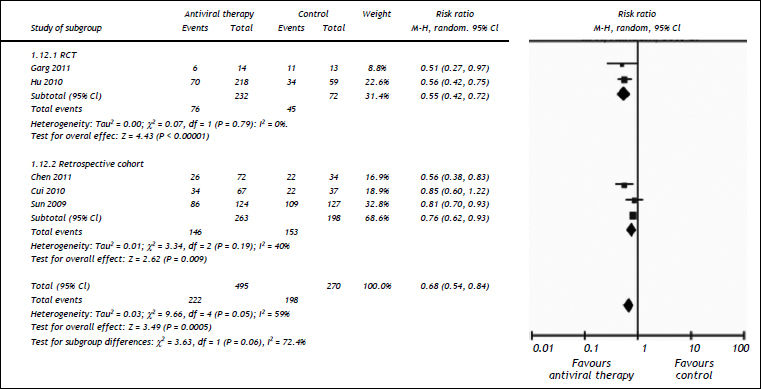
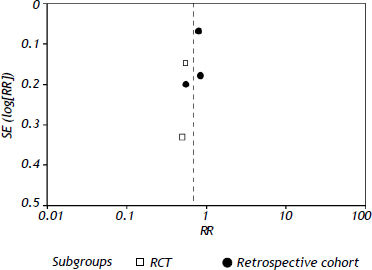
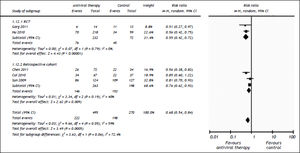
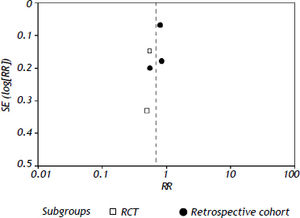
All the studies reported short-term mortality. Patients receiving nucleos(t)ide analogues had significantly lower short-term mortality than those in control group [44.8 vs. 73.3%, RR = 0.68, 95%CI (0.54, 0.84), P < 0.01] (Figure 2). Significant heterogeneity was observed among these studies (P = 0.05, I2 = 59%). No evidence of publication bias was found by either funnel plot (Figure 3) or Egger's test (P = 0.31). To confirm the stability of the primary analysis, we performed sensitivity analysis by excluding studies one-by-one, and found that the result did not change significantly with exclusion of any single study. Sub-group analysis showed that substantial heterogeneity might be due to varying study designs.
Comparison of the 3 month mortalities between nucleos(t)ide analogue and control groups of ACLF patients. Subgroup analysis regarding study designs (RCTs or retrospective cohorts) was performed. For each study, sample size and risk estimate were represented. Squares represent risk estimate of individual study; diamonds represent summary risk estimates; horizontal lines represent 95% confidence intervals. CI: confidence interval. A random effect model was used. An overall tendency towards the left side of the reference line (RR = 1) indicated that the antiviral therapy could reduce mortality.
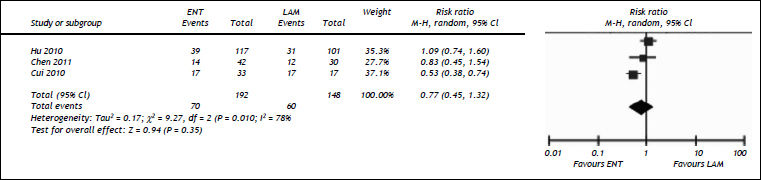
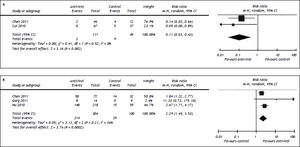
Three studies compared the efficacy of entecavir and lamivudine in rescuing the short-term survival of HBV-related ACLF patients.16–18 We found comparable short-term mortality between patients given with ENT and those with LAM [36.4 vs. 40.5%, RR = 0.77, 95%CI (0.45, 1.32), P = 0.35] (Figure 4). We observed significant heterogeneity among the included studies (P = 0.01, I2 = 78%). Egger’s test did not detect the presence of publication bias (P = 0.63).
Comparison of efficacy between entecavir and lamivudine in reducing 3 month mortality. For each study, sample size and risk estimate were represented. Squares represent risk estimate of individual study; diamonds represent summary risk estimates; horizontal lines represent 95% confidence intervals. ENT: entecavir. LAM: lamivudine. CI: confidence interval. A random effect model was used. The contact of overall diamond with the reference line (RR = 1) indicated that no difference in mortality between entecavir and lamivudine groups.
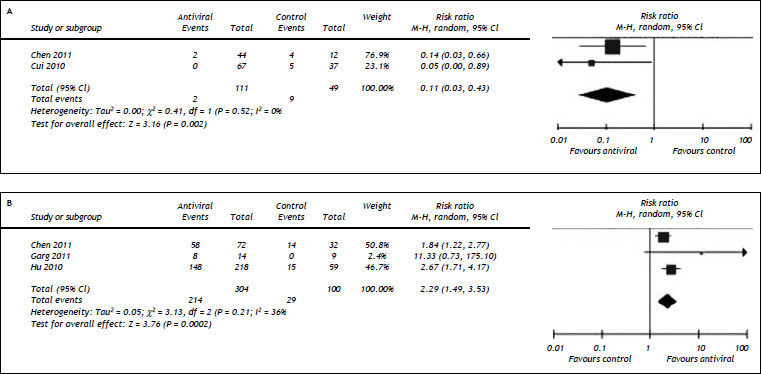
We also evaluated the impact of antiviral therapy on long-term recurrence of liver failure17,18 and short-term HBV DNA inhibition in HBV-related ACLF patients.15–17 Our results showed patients treated with nucleos(t)ide analogues had lower rate of reactivation [1.80 vs. 18.4%, RR = 0.11, 95%CI (0.03, 0.43), P < 0.01] (Figure 5A) and higher rates of profound HBV DNA reduction [HBV DNA reduction > 2 log10: 70.4 vs. 29%, RR = 2.29, 95%CI (1.49, 3.53), P < 0.01] (Figure 5B). No substantial heterogeneity was found with regard to reactivation and HBV DNA reduction. Potential publication bias was detected in comparison for HBV DNA reduction (P = 0.05) and not available in assessing pooled analysis for reactivation because of the small number of studies (n = 2).
Comparison of reactivation (A) and reduction of HBV DNA levels (B) between nucleos(t)ide analogue and control groups in ACLF patients. For each study, sample size and risk estimate were represented. Squares represent risk estimate of individual study; diamonds represent summary risk estimates; horizontal lines represent 95% confidence intervals. CI: confidence interval. A random effect model was used. An overall tendency towards the left (A) and right (B) sides of the reference line (RR = 1) indicated that the antiviral therapy could reduce ACLF reactivation and inhibit HBV DNA replication.
Four studies assessed the safety of nucleos(t)ide analogues in treating HBV-related ACLF.15,16,18,19 No study reported drug-related adverse events such as renal failure, pancreatitis and neuropathy.
No cases of YMDD mutation were reported within 3 month treatment period.
DiscussionRecently, the concept of ACLF was proposed as: ‘acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease’ by APSAL.11 The present study is the first meta-analysis to evaluate the efficacy and safety of nucleos(t)ide analogues in treating HBV-related acute-on-chronic liver failure. Our primary pooling result demonstrated that nucleos(t)ide analogue treatment reduced 3-month mortality of HBV-related ACLF patients. This was in line with a prior report that ACLF patients with high baseline viral load (HBV DNA ≥ 105 copies/mL) had poorer short-term prognosis than those with low viremia.20 Furthermore, even in the treatment group, only patients with a rapid decline of HBV DNA had a better prognosis.15,19 It suggested that viral factors participated in the pathogenesis of this severe hepatic necroinflammation and decompensation. Therefore, appropriate antiviral therapy might prevent or at least slow down the progression of liver necroinflammation and allow hepatic regeneration.
It was possible that this group of patients would benefit from more potent anti-HBV drugs which could decrease viral load in a more rapid manner. However, we found comparable efficacy between ENT and LAM in improving short-term prognosis, although ENT was demonstrated to be superior to LAM in suppressing HBV replication.21,22 It might be possible that the difference in the capacity of viral suppression between ENT and LAM was not large enough to impact on the prognosis of ACLF patients. Thus, it was worthwhile to compare TDF, which is a more potent drug, with other antiviral agents. Another important question was that when nucleos(t)ide analogues should be given. One of the included studies reported that LAM had no effectiveness in salvaging patients in advanced stages of ACLF (MELD score > 30),19 suggesting anti-HBV therapy should be given earlier.
Our secondary pooling results suggested necroinflamation analogues reduced long-term recurrence of HBV-related ACLF. This also has important clinical implications because those survived could not immediately return to their baseline status.23 Therefore, these cases are still at high risk of developing additional life-threatening flare-ups. Furthermore, it has been reported that recent previous hospitalizations were associated with a poorer prognosis for ACLF patients,23 underscoring the importance of preventing recurrence at least in the short-term. Therefore, sustained anti-viral therapy might be warranted for the benefit of long-term survival. However, the risk of antiviral resistance should be considered during long-term nucleos(t)ide analogue treatment as HBV reactivation might lead to additional life-threatening hepatic insults in these patients. Although antiviral-resistant mutations were not reported during follow-up in the included studies, the observation duration was too short to evaluate the incidence of antiviral resistance within extended nucleos(t)ide analogue therapy.1,2 Thus, further studies were needed to monitor the antiviral-resistant mutations in these patients.
Our meta-analysis has several strengths. Most importantly, to ensure the homogeneity of patient population, all the studies fulfilled the diagnostic criterion proposed by APSAL (4 studies directly adopted APSAL criteria and one within the criterion). In addition, the comparable 3-month mortality in controls (ranging from approximately 60% to 85.8%) suggested that the severity of ACLF in the patients in the different studies were similar. Second, most of the included trials were of high methodological quality.
However, there were also several limitations in this study. First, except super-infections of other hepatitis viruses, some studies did not exclude other causes that could constitute the precipitating events for the acute deterioration of hepatic function, such as sepsis, acute variceal bleeding, use of hepatotoxic drugs and herbal indigenous medicines. Therefore, whether all the HBV-related ACLF cases were initiated by HBV reactivation was not absolutely clear. Second, the limited sample size might weaken the validity of the conclusions. In addition, for retrospective cohort studies, there were possible unidentified confounders. For example, the application of artificial liver support systems was not mentioned in each study. Therefore, further large prospective studies were warranted to draw a definite conclusion. Third, all the included studies were conducted in Asian population, and therefore, the conclusion may not be generalized to other populations. Fourth, most studies did not evaluate the impact of nucleos(t)ide analogues on the longterm prognosis. It was reported that a considerable proportion of ACLF patients died after 3 months of antiviral therapy.24,25 Therefore, longer observation duration seemed to be necessary to determine the prognosis of these patients. Finally, we restricted our search to articles published in full text and published in English or Chinese. We might have omitted high-quality studies published in abstract or in other languages.
In conclusion, our results showed that antiviral therapy with nucleos(t)ide analogues reduced shortterm mortality as well as long-term recurrence of HBV-related ACLF patients. In addition, nucleos(t)ide analogues were well-tolerated during treatment period. However, these conclusions require further large prospective studies to be strengthened. Further studies investigating HBV-related ACLF should exclude other acute events precipitating hepatic decompensation. The long-term efficacy and safety (especially antiviral resistance) should be examined in future studies which should focus on the comparison among various nucleos(t)ide analogues to determine the optimal drug for this type of patients.
Abbreviations- •
ACLF: acute-on-chronic liver failure.
- •
ADV: adefovir.
- •
APSAL: Asian Pacific Association for the Study of the Liver.
- •
CHB: chronic hepatitis B.
- •
ENT: entecavir.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
LAM: lamivudine.
- •
LDT: telbivudine.
- •
TDF: tenofovir.
This work was supported by the 12-5 State S&T Projects of China (2012ZX10002007; 2012ZX10002004) and the National Natural Science Foundation of China (81100286).
Jianqin He contributes to this work equally.