Evaluation patients with nonalcoholic steatohepatitis (NASH) imply the need of appropriate assessment of disease severity (i.e. the presence of NASH) as well as of the disease stage (i.e. the extent of liver fibrosis). Liver biopsy (LB) is still considered the gold standard for diagnosing NASH as well as for establishing the degree of liver fibrosis. However, due to its invasive nature and costs, use of LB should be restricted to selected patients and, according guidelines and expert opinion, indicated in the following scenarios: a) when LB will guide treatment, b) to confirm or exclude NAFLD in patients with conflicting clinical data, c) to increase patient’s awareness about their disease, and improve engagement in their care and d) for inclusion in clinical trials. However, the role fo LB in NAFLD is evolving since when new and costly therapeutic agents become available, LB will be eventually necessary to make clinical decisions. The use of non-invasive tools (NITs) to assess steatosis, NASH and hepatic fibrosis is useful to triage NAFLD patients and decide in whom perform a LB.
Non-alcoholic fatty liver disease (NAFLD) spans a clinical-histological spectrum ranging from isolated steatosis to nonalcoholic steatohepatitis (NASH), a more aggressive form of the disease, consisting in a complex histological pattern that associates hepatocyte injury, inflammation and various degrees of liver fibrosis.1,2 NAFLD is tightly associated with cardiometabolic comorbidities including obesity, type 2 diabetes mellitus (T2DM), hypertension, and hypercholesteremia, which determines an increased risk of death linked to cardiovascular diseases, malignancy and T2DM complications.3 Those patients with NASH have also an increased liver-related mortality attributed to the progression to cirrhosis and its complications, including hepatocellular carcinoma (HCC).1,3 Of note, the extent of hepatic fibrosis has been shown to be the most robust predictor of liver related and possibly all-cause mortality in patients with NASH.3,4 For this reason, from the hepatologist’s point of view, to differentiate patients with isolated steatosis from those with more advanced disease (NASH and/or advanced fibrosis) is pivotal for a personalized management of the disease. In this context, the role of performing a liver biopsy (LB) in NAFLD patients has been matter of debate.4,5 Although histological assessment is considered the gold standard for diagnosing NASH,1,2,4 its role in clinical practice is evolving as alternative diagnostic tools are being developed and treatment decisions are modified due to the availability of new pharmacological interventions. In this short review, we discuss some clinical considerations on the use LB in subjects with NAFLD at present time and try to envision the evolving role of this diagnostic procedure in clinical decision-making when managing these patients.
Usefulness of Lb In Nafld: Diagnosis and PrognosisLB is a useful diagnostic tool to confirm or exclude NAFLD in patients with conflicting clinical data or competing etiologies of liver disease. Also, as mentioned above, LB is the most reliable way to precisely diagnose NASH1,4 and provides information about the presence and extent of liver fibrosis being therefore useful to assess patient prognosis since the presence of liver fibrosis is key in determining long-term liver-outcomes of NAFLD.1,6 With regard to steatohepatitis, there is some debate about its robustness in predicting long-term prognosis.7 Indeed, NASH have not been found to be associated to mortality in long-term studies. Of note, a general and biological plausible concept in liver diseases is that the necro-in-flammatory phenomena drive fibrogenesis in NAFLD and therefore steatohepatitis would a major factor behind disease progression in NAFLD.8,9 Moreover, several studies and meta-analysis have indeed shown that steatohepatitis is associated with a higher risk of fibrosis progression than isolated steatosis.10 Thus, it is likely that, due to methodological issues, existing studies failed to capture the association between NASH and long-term liver-related outcomes, independently of fibrosis stage.7 Therefore, histological evaluation of hepatocyte injury, inflammation and fibrosis does provide important information that may guide treatment strategies, stratify the risk of a given patient to develop advanced liver disease or establish the stage of the disease.4,11
It is of course important to consider that LB is an imperfect diagnostic tool with limitations related to sampling variability, inter- and intra-observer variability as well as the invasive nature of the procedure with its associated risks of morbidity and mortality.6,12 These limitations dictate that its use must be carefully decided considering the clinical setting and its impact in clinical management.1,2,11
Alternative Methods For Assessment of Nash and FibrosisIndeed, a “biopsy-all” approach is neither appropriate nor applicable for NAFLD patients in real life clinical practice due to accessibility, potential adverse events and associated costs. Moreover, since the majority of patients (70-75%) will present simple steatosis on liver histology, the number of patients needed to test in order to detect those with NASH and fibrosis would be very high. This could be reduced by selecting patients with high pre-test probability of having more severe disease. In this setting, non-invasive tools (NITs) can help to triage patients with NAFLD, avoid liver biopsy in a significant number of subjects and reduce costs and specialist referral. NITs include a number of tools including clinical prediction rules, direct measurement of markers of necroinflammation (i.e. measurement of circulating keratin 18 fragments) or fibrosis (i.e. assessment of Plasma collagen type III (Pro-C3) or determinations of proteins involved in fibrogenesis [FibroTestTM, the Enhanced Liver Fibrosis (ELFTM) test], composite predictive models (e.g. FIB-4 index, NAFLD fibrosis score [NFS], BARD score and others) and innovative imaging methods such as vibration controlled transient elastography (VTCE, FibroscanTM), acoustic radiation force imaging (ARFITM), shear wave elastography (SWE); or MRI based elastography (MRE). Besides evaluating NASH and fibrosis, some NITs are useful for steatosis quantification (i.e. coefficient attenuation parameter [CAP] and magnetic resonance imaging proton density fat fraction [MRI-PDFF]), which may also have prognostic importance.13
Although, these tools are very useful they should be conceived as an aid to select patients who should undergo LB rather than a replace of NAFLD histological assessment. The overall diagnostic accuracy of NITs has been recently reviewed14 and due to space constraints we can not analyze this aspect in detail. Suffice to say that there is no highly sensitive and specific tests to differentiate NASH from isolated steatosis and that the performance of NITs to rule in patients at risk is not as satisfactory as their ability to rule out severe liver fibrosis.
Intense research is being conducted to develop new tools for NASH diagnosis (i.e. metabolomics-based approaches such as the OWLiver testTM) or establish combinatorial approaches (i.e. combinations of clinical and blood-based biomarkers with new imaging techniques) to assess liver fibrosis that may accurately identify those patients at risk of progression and those who already progressed. These new approaches need to be evaluated in future studies. In the meantime, experts and current guidelines do recommend using the NFS or FIB-4 scoring systems as well as VCTE or MRE for identifying NAFLD patients with advanced fibrosis.1,14 In order to predict the presence of NASH in patients with NAFLD careful attention must be paid to the presence of metabolic syndrome components as well as to the coexistence of T2DM and obesity.1
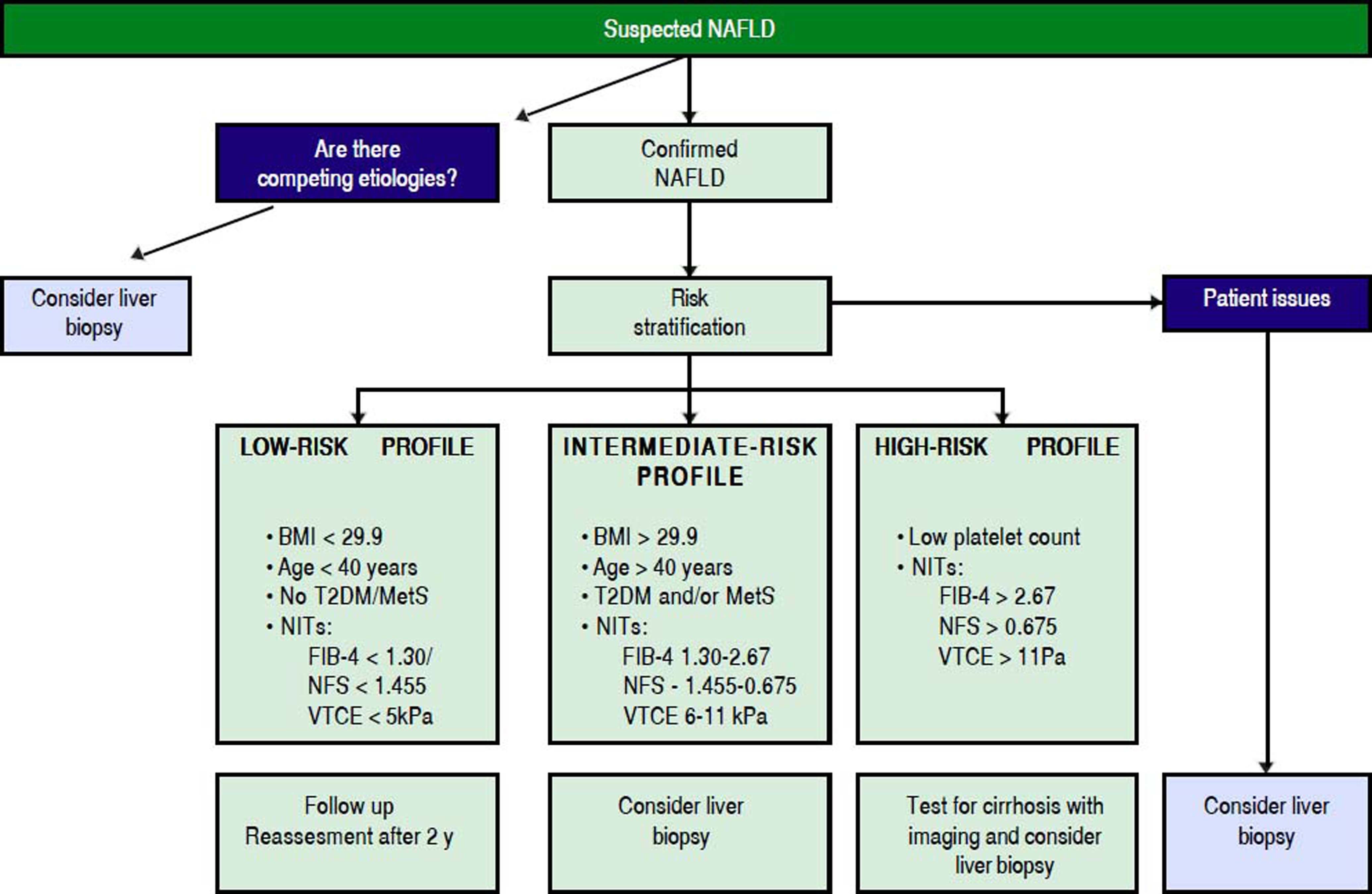
Inserting LB Into Patient Care AlgorithmAs mentioned, the role of LB in NAFLD management is evolving. The advent of NITs clearly has decreased the number of performed biopsies in recent years but when new, costly and potentially harmful therapies become available it is likely that, if NITs are still not completely validated or available, LB will be necessary to make clinical decisions. At present time, a liver biopsy should be performed in a patient with NAFLD in the following scenarios: a) it will guide treatment recommendations, b) to confirm or exclude NAFLD in patients with conflicting clinical data, c) to make patients aware about that they have a potentially serious liver disease and to increase patient engagement in their care and d) as requirement for recruitment in clinical trials.1,11 The use of NITs should be used to select patients for LB.14 In figure 1, a suggested algorithm for clinical decision is presented. However, more studies are needed to better define which is the best combination of NITs to more accurately select those patients at-risk of more severe outcomes.
Proposed algorithm to triage patients with NAFLD who need referral or further assessment and undergo liver biopsy for grading and staging of liver disease. Patient issues include lack of improvement of liver test after lifestyle changes, need of increasing patient’s awareness about their disease and engagement in their care and consideration of inclusion in clinical trials. BMI: body mass index. T2DM: type 2 diabetes mellitus. MetS: mtabolic syndrome. NITs: non-invasive tools. NFS: NAFLD fibrosis score. VTCE: vibration controlled transient elastography.
- •
ARFITM: Acoustic radiation force imaging.
- •
BARD: Body mass index Aminotransferase Ratio Diabetes mellitus.
- •
CAP: Coefficient attenuation parameter.
- •
FIB-4: fibrosis-4.
- •
HCC: hepatocellular carcinoma.
- •
LB: Liver biopsy.
- •
LB: Liver biopsy.
- •
MRE: MRI based elastography.
- •
MRI: Magnetic resonance imaging.
- •
MRI-PDFF: Magnetic resonance imaging proton density fat fraction.
- •
NAFLD: Non-alcoholic fatty liver disease.
- •
NASH: Nonalcoholic steatohepatitis.
- •
NFS: NAFLD fibrosis score.
- •
NITs: Non-invasive tools.
- •
SWE:Shear wave elastography.
- •
T2DM: Type 2 diabetes mellitus.
- •
VTCE, FibroscanTM: Vibration controlled transient elastography.
- •
ELFTM: Enhanced Liver Fibrosis.
The authors declares that there is no conflict of interest regarding the publication of this article.
AcknowledgmentsThis article was supported by research grants from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1150327 to M.A., and 1150311 to F.B.) and the Comisión Nacional de Investigación Científica y Tecnológica (grant CONICYT PIA/Basal PFB12, Basal Centre for Excellence in Science and Technology to M.A.) both from the Government of Chile.