Background and rationale for the study. The aim of the study was to determine the prognostic value of histopathological findings with special care to the severity of liver fibrosis at the moment of hepatopor-toenterostomy (HPE) in children with biliary atresia (BA). We performed analysis of 142 wedge liver biopsies taken at the time of HPE. All patients were operated by the same surgical team between 1995 and 2007. According to the outcome 6 months after HPE patients were divided into prognostic groups: group 1-bilirubin level < 2 mg% (n = 65), group 2-bilirubin level > 2 mg% (n = 77). Liver biopsies were re-evaluated according to the extended histopathological protocol and then were compared between the prognostic groups. Survival with native liver (SNL) estimates were performed in regard to severity of liver fibrosis.
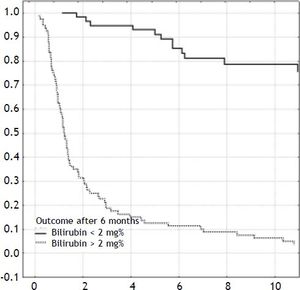
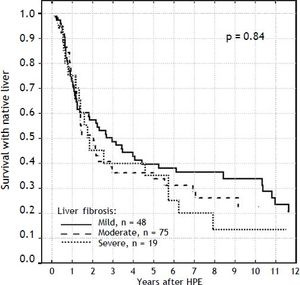
Results. Survival with native liver estimates after 2, 5 and 10 years in patients after successful operation were 96%, 91%, 75% vs. 30%, 11%, and 5% if operation failed (p < 0.001). There was no difference between groups in the following variables: fibrosis (p = 0.69), portal inflammation (p = 0.99), lobular inflammation (p = 0.95), cholangiolitis (p = 0.23), accumulation of bile pigments (zone 1:p = 0.49; zone 2:p = 0.51; zone 3:p = 0.48), bile plugs in canaliculi (p = 0.12), bile plugs in ducts (p = 0.32), bilirubinostasis in hepatocytes (p = 0.45), bile ductular proliferation (p = 0.59), ductal plate malformation (p = 0.12), focal necrosis (p = 0.44), giant cell transformation (p = 0.45), haematopoesis (p = 0.52), ductopenia (p = 0.46), microabscesses (p = 0.49), ballooning of hepatocytes (p = 0.08). The actuarial 5/10-year SNL was not dependent on severity of liver fibrosis (log-rank test p = 0.84). The severity of fibrosis corresponded neither with the age at HPE nor with the laboratory findings before operation but increased the risk of portal hypertension.
Conclusion. Liver histology at the time of HPE is of limited value in prognosis making in BA.
Biliary atresia (BA) is a progressive disease of extra and intrahepatic bile ducts developing in the first weeks of life, with varying occurrence between 1:5,000 and 1:20,000.1–3 It is the main cause of neonatal cholestasis and most frequent indication to liver transplantation (LTx) in children, representing over 50% of pediatric cases.4 The treatment of choice is Kasai hepatoportoenterostomy (HPE) which is a resection of fibrous remnants of biliary ducts followed by anastomosing a conventional Roux-en-Y loop of jejunum to the fibrous edges of the transected fibrous tissue in the porta hepatis.5 The restoration of intestinal bile flow within the first months after surgery may delay or even stop the progression of disease. Nevertheless, progressive devastating process inevitably leads to LTx in most of the affected children. The pathways of ongoing injury, so as the etiology of disease remain unclear. Pathological inflammatory reaction to some extrinsic factor and genetic predisposition are considered most likely.6 Liver histology usually presents with specific features discriminating BA from other causes of neonatal cholestasis in the majority of cases. Typically, varying degree of cholestasis, inflammation, fibrosis and ductular proliferation is present.2,6 How far severity of these changes predicts outcome of treatment was analyzed previously but with inconsistent conclusions.
The aim of this study was to perform thorough analysis of histopathological presentation in children with BA and to correlate it with an early and long-term outcome, with special emphasis on liver fibrosis.
Material and MethodsWe performed the retrospective chart review of 142 children (86 female/56 male) with BA type III (complete obliteration) who underwent HPE in our institution between 1995 and 2006. The operative wedge liver biopsy was performed in each case. Early outcome was based on the restoration on bile flow and total bilirubin (TB) concentration within 6 months after HPE. Liver biopsies were re-evaluated according to the detailed histopathological protocol and than were compared between the outcome groups. The severity of liver fibrosis was analyzed in regard to survival with native liver, age at HPE, laboratory findings and development of portal hypertension.
All patients received standard postoperative treatment including antibiotic prophylaxis for 6-12 months, ursodeoxycholic acid, nutritional and vitamin supplementation. Steroids were used in some proportion of patients but doses and periods of treatment changed over time what precluded estimation of its effects on overall outcome. Patients were under regular checks in outpatient clinics. Any suspicion of portal hypertension development was followed by endoscopy. Varices were eradicated by EVL (oesophagal variceal ligaton) in all patients with no regard to previous history of bleeding.
Statistical assessment was based on Fisher exact test, Kruskal Walis test and log-rank test with Kaplan Meier survival estimates as appropriate. The end-point for survival analysis was death, listing for LTx or LTx. The research was approved by Institutional Ethical Board.
Histological examinationThe liver wedge biopsies from the right lobe were collected during Kasai procedure. Each biopsy was assessed by two experienced pathologist blinded to clinical outcomes. All liver biopsy specimens were fixed in 10% neutral buffered formalin pH 7.4 for 12-24 hours and embedded in paraffin and cut into 4 μm thick sections. Sections displaying at least 10 portal tracts were routinely stained by hematoxylineosin, periodic Acid-Schiff method, with and without digestion, Gomori silver stain and Azan method. The ductal and ductular phenotype was examined on paraffin embedded tissue using monoclonal antibodies directed against Cytokeratin 7 (OV-TL 1:50 Dako) and 19 (RCK 108 1:100 Dako) and SMA by means of EnVision system DAKO. This method allowed to determine changes specific for ductal plate malformation by the positive staining on bile ducts epithelia and abnormal ductal structures. The cells positive for CK7 were recognized as bile ductular epithelial cells even if they did not show any tubular structure. In some cases, a few liver cells in the lobule showed a positive reaction for CK7.
Histological criteriaIn the course of evaluation histological activity of the disease, each sample was described for fibrosis and inflammation. The fibrosis scores were defined after Ishak as follows:7
- •
0: no fibrosis.
- •
1: fibrous expansion of some portal areas, with or without short fibrous septa.
- •
2: fibrous expansion of most portal areas, with or without short fibrous septa.
- •
3: fibrous expansion of most portal areas with occasional portal to portal bridging.
- •
4: fibrous expansion of most portal areas with marked bridging (portal to portal as well as portal to central).
- •
5: marked bridging with occasional nodules (incomplete cirrhosis).
- •
6: cirrhosis, probable or definite.
Inflammatory changes in portal tracts were classified as grade:
- •
0: no inflammation
- •
1: minimal inflammation.
- •
2: mild inflammation (cells in less than one third of portal tracts).
- •
3: moderate inflammation (cells in one third to two thirds of portal tracts).
- •
4: severe inflammation (dense packing of cells in more than two thirds of portal tracts).
Inflammatory changes in lobules, bile stasis in hepatocytes and bile plugs in canaliculi were assessed as absent (0), minimal (+1), mild (+2), moderate (+3) and severe (+4). The following categories of lesions were assessed as present or absent: bile duct proliferation, bile stasis in zones 1-3, cholangiolitis, microabscesses, focal necrosis, giant cell transformation, bile plugs in ducts, hematopoiesis, ductopenia and balooning of hepatocytes. Presence or absence of malformed ductal plate structures was recorded according to the Desmet description.8
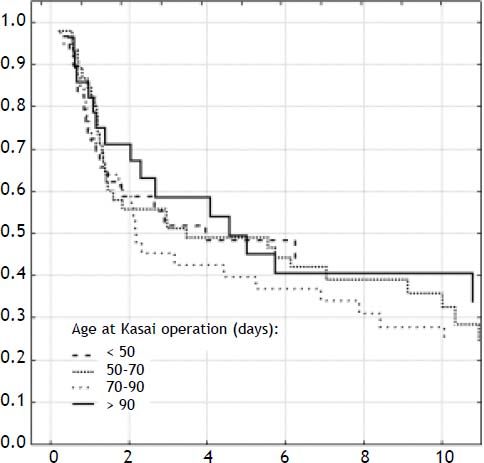
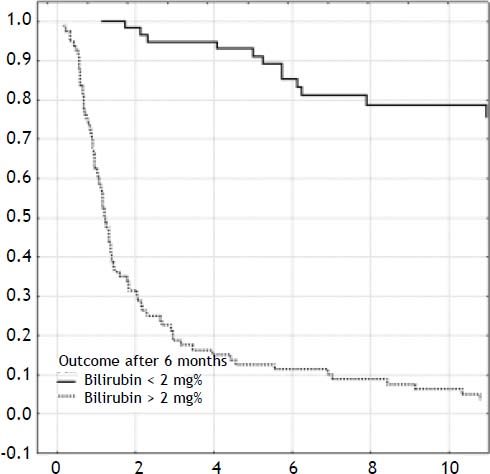
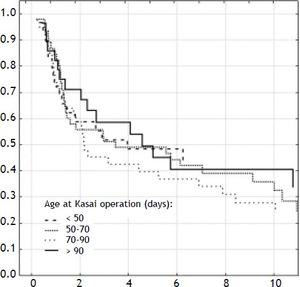
ResultsOverall outcomeOne-hundred-forty-two neonates with BA type III underwent HPE at the median age of 69 days (27-131). The overall actuarial 5 and 10-year survival with native liver were 38 and 19% respectively, and did not show significant differences according to the age at surgery, p = 0.61 (Figure 1). An early outcome was based on the restoration of bile flow and reduction of total bilirubin (TB) concentration after HPE as a most reliable predictor of outcome (Figure 2). Survival with native liver estimates after 2, 5 and 10 years in patients after successful operation were 96%, 91%, 75% vs. 30%, 11%, and 5% if operation failed (p < 0.001). Due to this observation patients were grouped according to bilirubin 6 month after HPE:
- •
Good outcome group: total bilirubin level < 2 mg% (n = 65).
- •
Poor outcome group: total bilirubin level > 2 mg% (n = 77).
Histopathologic assessment was performed and compared between groups accordingly.
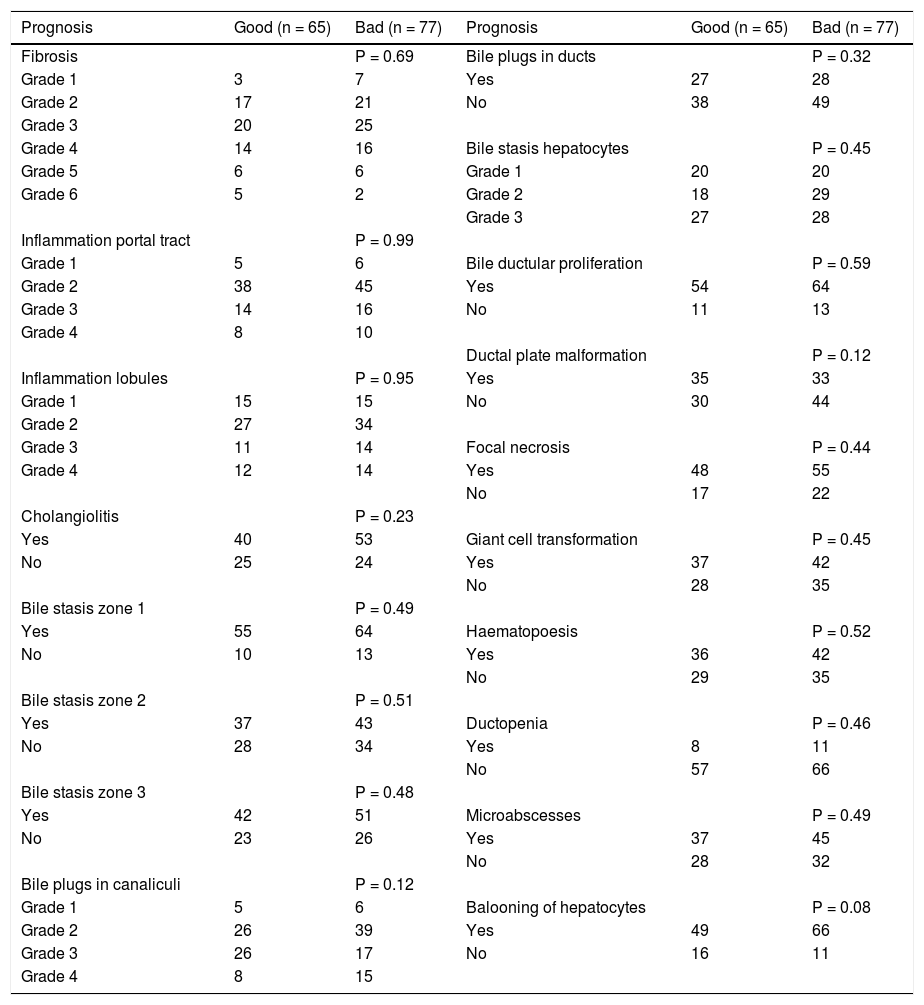
Histological presentationIn general, histological assessment did not show significant differences between prognostic groups. All variables are summarized in table 1. Nevertheless there were differences in severity of particular parameters.
Correlation of histopatology between prognostic groups within 6 months after Kasai hepatoportoenterostomy.
| Prognosis | Good (n = 65) | Bad (n = 77) | Prognosis | Good (n = 65) | Bad (n = 77) |
|---|---|---|---|---|---|
| Fibrosis | P = 0.69 | Bile plugs in ducts | P = 0.32 | ||
| Grade 1 | 3 | 7 | Yes | 27 | 28 |
| Grade 2 | 17 | 21 | No | 38 | 49 |
| Grade 3 | 20 | 25 | |||
| Grade 4 | 14 | 16 | Bile stasis hepatocytes | P = 0.45 | |
| Grade 5 | 6 | 6 | Grade 1 | 20 | 20 |
| Grade 6 | 5 | 2 | Grade 2 | 18 | 29 |
| Grade 3 | 27 | 28 | |||
| Inflammation portal tract | P = 0.99 | ||||
| Grade 1 | 5 | 6 | Bile ductular proliferation | P = 0.59 | |
| Grade 2 | 38 | 45 | Yes | 54 | 64 |
| Grade 3 | 14 | 16 | No | 11 | 13 |
| Grade 4 | 8 | 10 | |||
| Ductal plate malformation | P = 0.12 | ||||
| Inflammation lobules | P = 0.95 | Yes | 35 | 33 | |
| Grade 1 | 15 | 15 | No | 30 | 44 |
| Grade 2 | 27 | 34 | |||
| Grade 3 | 11 | 14 | Focal necrosis | P = 0.44 | |
| Grade 4 | 12 | 14 | Yes | 48 | 55 |
| No | 17 | 22 | |||
| Cholangiolitis | P = 0.23 | ||||
| Yes | 40 | 53 | Giant cell transformation | P = 0.45 | |
| No | 25 | 24 | Yes | 37 | 42 |
| No | 28 | 35 | |||
| Bile stasis zone 1 | P = 0.49 | ||||
| Yes | 55 | 64 | Haematopoesis | P = 0.52 | |
| No | 10 | 13 | Yes | 36 | 42 |
| No | 29 | 35 | |||
| Bile stasis zone 2 | P = 0.51 | ||||
| Yes | 37 | 43 | Ductopenia | P = 0.46 | |
| No | 28 | 34 | Yes | 8 | 11 |
| No | 57 | 66 | |||
| Bile stasis zone 3 | P = 0.48 | ||||
| Yes | 42 | 51 | Microabscesses | P = 0.49 | |
| No | 23 | 26 | Yes | 37 | 45 |
| No | 28 | 32 | |||
| Bile plugs in canaliculi | P = 0.12 | ||||
| Grade 1 | 5 | 6 | Balooning of hepatocytes | P = 0.08 | |
| Grade 2 | 26 | 39 | Yes | 49 | 66 |
| Grade 3 | 26 | 17 | No | 16 | 11 |
| Grade 4 | 8 | 15 |
The degree of fibrosis varied in the examined material, but predominated moderate fibrosis. Mild fibrosis (degree 1 and 2) was present in both groups (31% of patients with good vs. 36% with poor prognosis) after 6 months. Moderate fibrosis (grade 3 and 4) was the same in both groups (53% of patients with good vs. 53% with poor prognosis) after 6 months and surprisingly severe fibrosis (grade 5 and 6) occurred more frequently in the group of good prognosis after 6 months (17% vs. 10%).
Ductular proliferation was the same in both groups after 6 months (83% vs. 83%). In 13% of all patients we observed signs of ductopenia. Ductal plate malformation was observed more frequently in the group of patients with good prognosis (53% vs. 42%).
Non-specific inflammatory infiltrates in the portal tracts were always present and similar with both groups, in contrast to the inflammatory reaction around bile ducts, which occurred in about 61% vs. 68%. We also observed small foci of inflammation in the lobules of all patients but focal necrosis occurred in 71% vs. 71% and 73% vs. 72% of patients with good vs poor prognosis respectively. Microabscesses, as sign of surgical hepatitis, were seen in 75% of patients with good vs. 86% with poor prognosis. The incidence of ballooning of hepatocytes was higher in group 2 and almost reached significance(p = 0.08). This is however non-specific parameter and interpretation should be cautious.
Severity of liver fibrosis and survival with native liverFor the further analyses, the liver fibrosis was arranged into groups according to Ishak scale:
- •
Grades 1-2: mild (n = 48).
- •
Grades 3-4: moderate (n = 75).
- •
Grades 5-6: severe (n = 19).
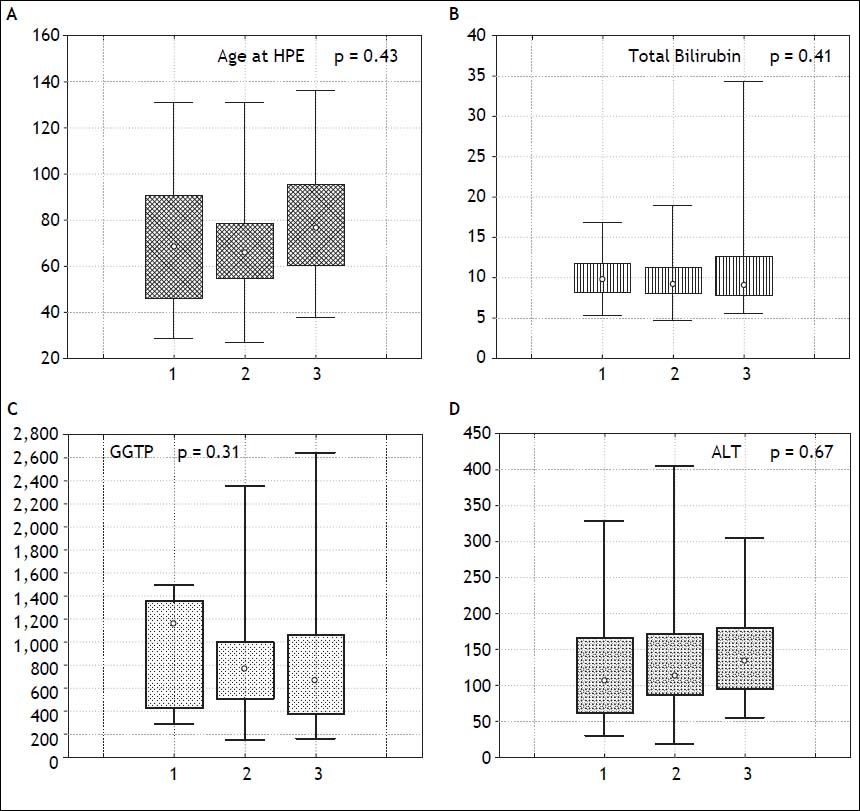
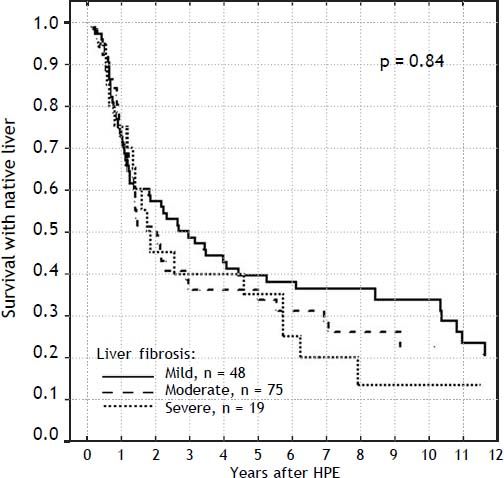
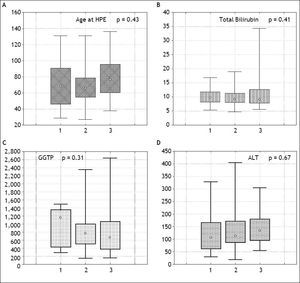
The severity of fibrosis corresponded neither with age at HPE (median age of 69 days range 27-131) nor with the laboratory findings before operation (mean total bilirubin 10.1 mg% (SD ± 3.4), ALT 134 (SD ± 70) and GGTP 847 (SD ± 514) (Figure 3). Estimates of SNL were the worst in children with severe fibrosis or cirrhosis in the initial biopsy, however differences were not significant statistically. Probability of 5/10-year SNL in mild, moderate and severe fibrosis group was: 36/23, 39/33 and 35/ 13% respectively, p = 0.84 (Figure 4).
Significant portal hypertension (SPH) was defined by at least one of the following: variceal bleeding, esophageal varices at least II grade or with red mark spots, or gastric varices. SPH developed in 57 (40%) and variceal bleeding occurred in 37 (26%). Severity of fibrosis correlated with SPH and developed in 6 (11%) with mild, in 35 (47%) with moderate and in 16 (76%) patients with severe fibrosis or cirrhosis.
DiscussionIn spite of the fact that in most patients HPE serves as a bridge to liver transplantation, still 20-year survival with native liver may be expected in over 20% of patients.9 The principal condition of favorite long-term outcome is restoration of bile flow after HPE reflected by significant drop in serum bilirubin concentration.10 The outcome usually correlated with the age at operation, experience of surgical team and anatomical type of BA.2,11 In our analysis we eliminated this variables. Age at operation was not significant as prognostic factor, all patients had type III - complete obliteration and on average, fifteen Kasai operations are performed annually in our institution.
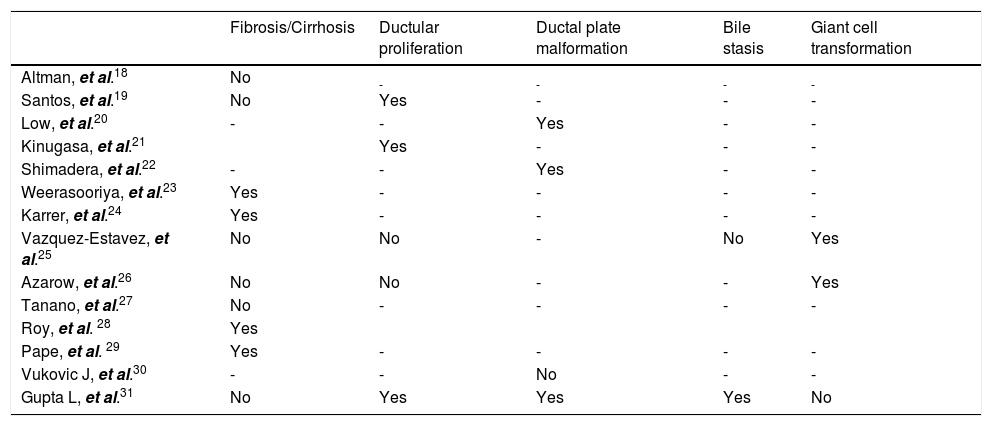
Liver biopsy, routinely taken during HPE, naturally brought the concept of its importance in the assessment of prognosis. Characteristic features comprise ductular proliferation (83 %), moderate fibrosis (53%) with absence of sinusoidal fibrosis and bile stasis and ductal plate malformation in 42 up to 53% of patients.12 Proportions of these changes may evolve over time however, from predominant portal inflammation and features of ductular obstruction at the beginning stages to more fibrotic state with destruction of bile ducts later on.13–17 Individual dynamics of histological presentation carries significant clinical implications. First is that samples taken before 6 weeks of age may be inconclusive and repeated liver biopsy may be required for diagnosis.17 Second, there is a difficulty in the assessment of prognostic value of histological features what may account for inconsistent results in previous studies summarized in table 2.18–31
Summary of studies in biliary atresia involving liver biopsy parameters as prognostic factors.
| Fibrosis/Cirrhosis | Ductular proliferation | Ductal plate malformation | Bile stasis | Giant cell transformation | |
|---|---|---|---|---|---|
| Altman, et al.18 | No | - | - | - | - |
| Santos, et al.19 | No | Yes | - | - | - |
| Low, et al.20 | - | - | Yes | - | - |
| Kinugasa, et al.21 | Yes | - | - | - | |
| Shimadera, et al.22 | - | - | Yes | - | - |
| Weerasooriya, et al.23 | Yes | - | - | - | - |
| Karrer, et al.24 | Yes | - | - | - | - |
| Vazquez-Estavez, et al.25 | No | No | - | No | Yes |
| Azarow, et al.26 | No | No | - | - | Yes |
| Tanano, et al.27 | No | - | - | - | - |
| Roy, et al. 28 | Yes | ||||
| Pape, et al. 29 | Yes | - | - | - | - |
| Vukovic J, et al.30 | - | - | No | - | - |
| Gupta L, et al.31 | No | Yes | Yes | Yes | No |
The severity of liver fibrosis, as a major indicator of disease progression, was most commonly analyzed parameter. We showed it did not correspond with the outcome of HPE, however there was only 13% probability of long-term survival in case of advanced liver fibrosis or cirrhosis at the moment of operation. There was no correlation between age at HPE and severity of fibrosis, what is contradictory to previous reports.23
Pape, et al.29 calculated the mean volume of fibrosis per number of periportal fields (Vfib) and the Ishak score. Detection of Vfib at the time of HPE was proved to be a valid marker in predicting transplant-free survival in children with BA however the Ishak score showed no correlation with transplant-free survival or Vfib. After extensive analysis, Lampela, et al. did not find correlation between severity of neither fibrosis nor inflammation and survival with native liver, however proportions of inflammatory components showed more lymphocytes and less macrophages in those who were transplanted within 2 years. Moreover none of the histologic variables at HPE predicted development of esophageal varices.15 Our data showed that risk of portal hypertention increases with severity of liver fibrosis.
Ductal plate malformation structures (DPM), are the remnants of impaired remodeling of primitive fetal bile ducts during organogenesis.32 These structures are found in other pathologies as well but in BA they may be associated with poor prognosis.20,22,31
Ductular proliferation usually seen in BA, results from uncontrolled response to chronic cholestasis and supposedly originates from a proliferation of preexisting intrerlobular bile ducts and ductules or from ductular transformation of the periportal hepatocytes.33,34 Kinugasa, et al.21 estimated intensity of ductular proliferation by cytokeratin-7 (CK-7) staining. The number of CK7-positive cells in the bile ductules was microscopically calculated within the 40-μm-thick interstitium along the limiting plate (LP), and the CK7-positive cell number per unit length of the LP was estimated. The higher number of CK7-positive cells correlted with poor bile drainage after HPE. In other paper the presence of syncytial giant cells, lobular inflammation, focal necrosis, bridging necrosis and cholangitis were each associated with failure of HPE and bile in zone 1 with success of operation.26
Discrepancies in current evidence make prognostic value of histological assessment unclear. Moreover, subjectivity of examination may bring additional bias. That issue was raised by biliary atresia research consortium (BARC), which identified bile plugs in ducts, giant-cell transformation, extramedullary hematopoiesis, and bile duct proliferation as most reliable features in terms of interobserver agreement.15
The major limitation of our study is retrospective nature of collected data, but liver specimens were re-examined in accordance to the newly prepared protocol. We also lacked histopathology from longterm native liver survivals since we do not perform liver biopsies in these patients routinely.
We concluded that liver histology at the time of HPE is of limited value in prognosis-making and should be interpreted cautiously in regard to the other clinical factors. Patients with severe fibrosis or cirrhosis in initial biopsy are likely to develop portal hypertension.
AcknowledgementsWe would like to thank Mrs. Krystyna Lech-Kunkel for major contribution in coordination of the study and specimen management.