Nonalcoholic fatty liver disease (NAFLD) is an alarming public health problem. The disease is one of the main causes of chronic liver disease worldwide and is directly linked to the increased prevalence of obesity and type 2 diabetes mellitus (T2DM) in the general population. The worldwide prevalence of NAFLD has been estimated at 20-30%, but the prevalence is unknown in the Americas because of a lack of epidemiological studies. However, given the trends in the prevalence of diabetes and obesity, the prevalence of NAFLD and its consequences are expected to increase in the near future. The aim of the present study is to present the current data on the prevalence of NAFLD in the Americas. We performed an electronic search of the main databases from January 2000 to September 2013 and identified 356 reports that were reviewed. We focused on the epidemiology and prevalence of known NAFLD risk factors including obesity, T2DM, and the metabolic syndrome (MS). The prevalence of the MS was highest in the United States, Mexico, Costa Rica, Puerto Rico, Chile, and Venezuela. In addition, Puerto Rico, Guyana, and Mexico have the highest prevalence of T2DM in the Americas, while USA has the most people with T2DM. In conclusion, the prevalence rates of NAFLD and obesity were highest in the United States, Belize, Barbados, and Mexico.
Nonalcoholic fatty liver disease (NAFLD) refers to the presence of hepatic steatosis, as demonstrated by imaging or by histology, in the setting of no significant alcohol consumption and the absence of other known secondary causes.1 NAFLD is strongly associated with obesity and metabolic disturbances such as insulin resistance (IR), type 2 diabetes mellitus (T2DM), and dyslipidemia. In the past few decades, obesity has become an epidemic health problem and, as a consequence, we are witnessing an increase in the prevalence of NAFLD in all age groups. NAFLD has become the most frequent cause of chronic liver disease in Western countries.2 Because the populations in the Americas seem to be particularly susceptible to obesity and T2DM, and because of the increasingly inactive lifestyles with abundant and high-energy food, NAFLD is expected to become a highly prevalent disorder in this world region.3
NAFLD is associated with increases in all-cause and liver-related mortality compared with the general population, in fact cardiovascular disease is the most common cause of death in NAFLD patients. Thus, the diagnosis, treatment, and prevention of NAFLD and concomitant metabolic disorders in these individuals are of high priority to health systems in the Americas.1,4 The aim of this review is to examine the available information on the prevalence of NAFLD and/or related disorders in the Americas and to generate good estimates of the magnitude of the problem in this continent.
Definition and natural history of NAFLDNAFLD is defined as the presence of lipid accumulation within hepatocytes accounting for > 5% of the total liver weight (mainly in the perivenular region and periportal areas)5 together with no evidence of significant alcohol consumption (defined as > 21 drinks per week in men or > 14 drinks per week in women over the preceding 2-year period, or > 10 g of daily alcohol consumption at the current time), and no current treatment with steatogenic medication or presence of a dietary disorder.1
Clinically, NAFLD comprises a broad pathological spectrum that includes simple steatosis; fatty liver accompanied by inflammation, fibrogenesis, and hepatocellular necrosis (nonalcoholic steatohepatitis, NASH); increased fibrosis and the consequent loss of liver function (cirrhosis); and, in some cases, development of hepatocellular carcinoma (HCC).1,5
In 10-20% of NAFLD patients with simple fatty liver disease, the disease will progress to NASH, and 3-5% might develop cirrhosis.5 The prevalence of HCC in cirrhotic patients with NASH is 2-3% per year; cirrhotic patients have a lower risk of developing HCC compared with those infected with hepatitis C virus.6,7
DiagnosisMost NAFLD patients are asymptomatic, and the diagnosis often follows abnormal findings from routine biochemistry tests, an abdominal ultrasound, or assessment of cardiovascular risk.8
NAFLD screening is controversial in asymptomatic and even in high-risk patients.9 Although liver biopsy is the gold standard for the diagnosis of NAFLD and NASH, this method has several disadvantages such as cost, invasiveness, and dependence on the operator’s skill, and the fact that because the histological features (e.g., inflammation and hepatocyte ballooning) are distributed unequally throughout the liver parenchyma, the disease can be misdiagnosed. In addition, diagnosis based on pathology results is often not possible in community-based research studies and clinical practice settings.1
Alternatively, several noninvasive markers or tests have been proposed for diagnostic purposes including biochemical criteria (aminotransferase levels) and hepatic imaging (ultrasonography, computed tomography, and magnetic resonance imaging), however these markers and tests have limitations.
Mild to moderate elevation of serum aminotransferase levels is the most frequent and often the only laboratory abnormality found in patients with NAFLD; however, most people with NAFLD have normal aminotransferase levels.10 Thus, studies using liver enzyme measures probably underestimate the prevalence of NAFLD. The best noninvasive method for diagnosing steatosis seems to be ultrasound (US).9 However, US has one important disadvantage: low accuracy in people with NAFLD and other conditions such as high body mass index (BMI) or steatosis < 10% of liver weight.11 Proton magnetic resonance spectroscopy (1H MRS) is better than US in detecting and quantifying minor fat infiltration, and it can detect steatosis as low as 3% of total liver weight.12,13
A number of biomarkers have been developed to differentiate between simple steatosis and NASH: blood cytokeratin-18 fragment, adipocyte fatty acid binding protein, and fibroblast growth factor 21.14,15,16 Although these biomarkers hold great promise as noninvasive markers of NASH, they must be confirmed in independent cohorts to achieve better diagnostic accuracy.
EpidemiologyThe epidemiology of NAFLD is an intense area of research worldwide. Several studies have reported a prevalence of 20-30% in unselected populations from developed countries7,10,17 and a prevalence of 2.7-12.2% for NASH.18 In a recent prospective cohort study of asymptomatic middle-aged patients using US and liver biopsy, the prevalence rates of NAFLD and NASH were reported as 46% and 12.2%, respectively.18,19 The incidence has been estimated at one in three adults in the developed world,20 Europe, parts of Africa, and Asia.2 NAFLD can affect individuals at any age.
In the Americas, most studies of NAFLD have focused on specific groups instead of the general population, and there are few studies on the overall prevalence of NAFLD in specific countries or regions. Hence, the exact incidence and prevalence throughout the continent remains unknown. The prevalence of NAFLD is expected to increase in the Americas given the socioeconomic and demographic transitions over the past 20 years and the associated unhealthy dietary habits, acquisition of an industrial urban lifestyle, and changes in epidemiological profile of disease prevalence, especially in South America.21 There has been an increase in the prevalence of chronic noncommunicable diseases, mainly cardiovascular disease and cancer, because of the increases in longevity and decreases in the prevalence of infectious diseases.21–24
The demographic transition in Mexico has been marked by shifts from high to low fertility and mortality, and by population aging. Nutrition has shifted from a high prevalence of undernutrition to a predominance of diet-related noncommunicable chronic diseases. These changes are associated with rapid processes of urbanization and economic growth because of technological changes and innovations, which have led to reduced physical activity levels and changes in food patterns and dietary intake, including increased consumption of energy-dense processed foods.25
In this context, health interventions to prevent or manage chronic diseases become important because of the 10 leading causes of mortality in adults in the Americas, seven are chronic diseases.26
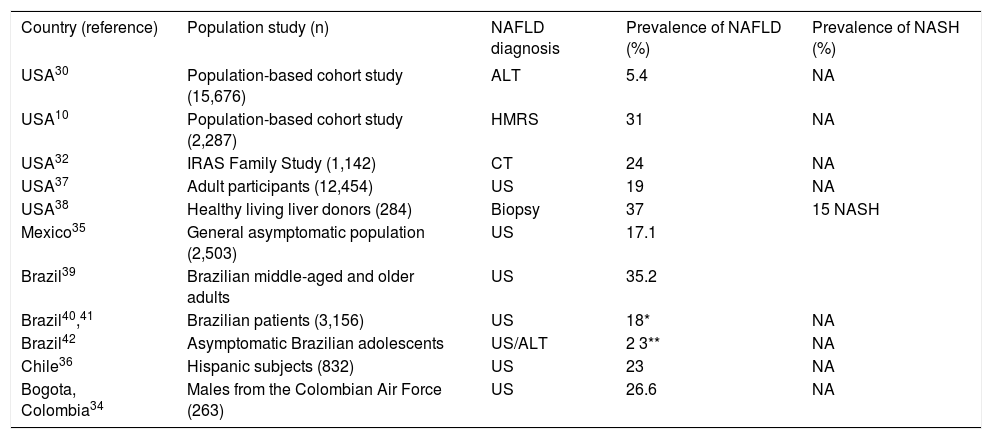
The prevalence of NAFLD in the Americas has been investigated using different diagnostic methods, and varies depending on the screening test used and the specific population studied. Table 1 summarizes the results of some studies that have published the prevalence of NAFLD in the United States (USA) and Latin American countries. Interpretation of these findings should take into account the source population and the operational definition of NAFLD used in the studies.
Prevalence of NAFLD in some countries.
| Country (reference) | Population study (n) | NAFLD diagnosis | Prevalence of NAFLD (%) | Prevalence of NASH (%) |
|---|---|---|---|---|
| USA30 | Population-based cohort study (15,676) | ALT | 5.4 | NA |
| USA10 | Population-based cohort study (2,287) | HMRS | 31 | NA |
| USA32 | IRAS Family Study (1,142) | CT | 24 | NA |
| USA37 | Adult participants (12,454) | US | 19 | NA |
| USA38 | Healthy living liver donors (284) | Biopsy | 37 | 15 NASH |
| Mexico35 | General asymptomatic population (2,503) | US | 17.1 | |
| Brazil39 | Brazilian middle-aged and older adults | US | 35.2 | |
| Brazil40,41 | Brazilian patients (3,156) | US | 18* | NA |
| Brazil42 | Asymptomatic Brazilian adolescents | US/ALT | 2 3** | NA |
| Chile36 | Hispanic subjects (832) | US | 23 | NA |
| Bogota, Colombia34 | Males from the Colombian Air Force (263) | US | 26.6 | NA |
ALT: alanine aminotransferase. NA: not applicable. HMRS: magnetic resonance spectroscopy. CT: computed tomography. US: ultrasound.
NAFLD is the most frequent cause of abnormal liver biochemistry findings in many developed and developing countries.27,28 In the USA, NAFLD seems to be more prevalent when diagnosed by 1H MRS and liver biopsy than by aminotransferase levels. In a study of the frequency of elevated aminotransferase levels as a surrogate for NAFLD and the MS in overweight multiethnic children/adolescents from South and Central America and the Caribbean, Hispanics had higher enzyme levels than Afro-Caribbeans.29
Several population studies have been performed to estimate NAFLD prevalence in the USA. A cohort study showed that 2.8-5.4% have abnormal liver enzyme levels unrelated to alcohol, viral hepatitis, or iron overload17,30 and that 66-90% of such cases are probably secondary to NAFLD.31 However, this method for NAFLD diagnosis has low specificity for detecting NAFLD. Another study using 1H MRS in all participants in the Dallas Heart Study (32.1% Caucasians, 48.3% African Americans, and 17.5% Hispanics) concluded that the prevalence of hepatic steatosis varies significantly with ethnicity (45% in Hispanics; 33% in whites; 24% in African Americans). The differences in the prevalence of NAFLD also appeared to be related to the higher prevalence of obesity and IR in Hispanics. They also reported that one in three adult Americans and one in 10 children/adolescents have steatosis, which equates to > 70 million adult Americans with NAFLD.10
Another study that examined risk factors for and heritability of NAFLD found that NAFLD was more common in Hispanic Americans than in African Americans. Insulin sensitivity and visceral adipose tissue correlated independently with NAFLD to a similar extent in the two ethnic groups. Age, triglyceride level, and plasminogen activator inhibitor 1 level were also significantly associated with NAFLD in Hispanics, whereas adiponectin was independently associated with NAFLD only in African Americans.32
The prevalence of NAFLD has been studied in some Latin American countries. In a multicenter analysis of 1,280 patients in Brazil, liver biopsy was performed in 437 patients, and isolated steatosis, steatohepatitis, and fibrosis were found in 42%, 58%, and 27% of these patients, respectively.33 A cross-sectional study in Bogota, Colombia, reported a prevalence of 26.6% of NAFLD in the young male Hispanic population.34 A cohort study of 2,503 Mexican patients who underwent a medical examination35 and a study of the general population in Chile,36 both of which used US to diagnose NAFLD, found prevalence rates of 17.1% and 22%, respectively10,30,32,34–42 (Table 1). From the data published to date, it appears that the highest prevalence is in the USA, where biopsy is used for diagnosis, whereas the prevalence is lower in Brazil, where US is used.
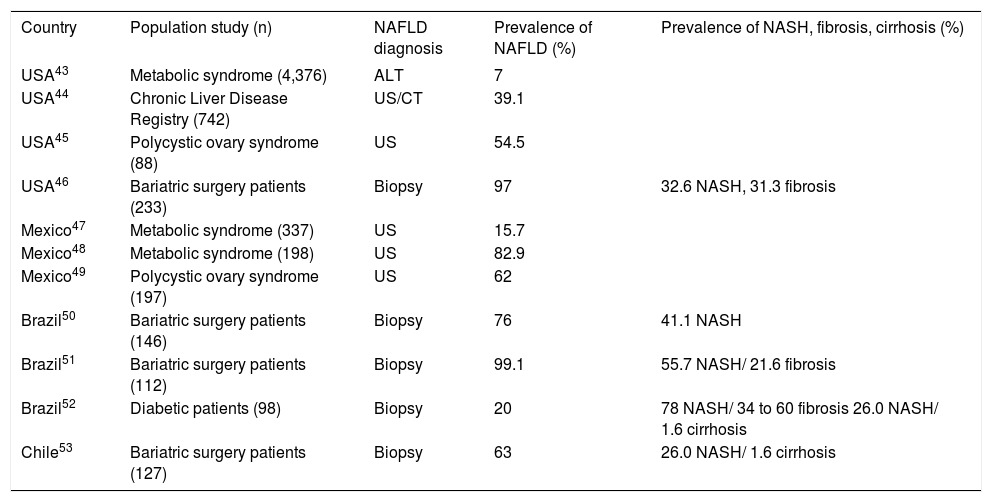
Table 243–53 summarizes the results of several studies on the prevalence of NAFLD in high-risk populations by country. These studies show that the prevalence is as high as 99% in groups with common risk factors such as T2DM, obesity, and/or the MS, and in specific high-risk groups such as those with morbid obesity or polycystic ovary syndrome.
Studies conducted on the prevalence of NAFLD in high risk populations in several countries across the Americas.
| Country | Population study (n) | NAFLD diagnosis | Prevalence of NAFLD (%) | Prevalence of NASH, fibrosis, cirrhosis (%) |
|---|---|---|---|---|
| USA43 | Metabolic syndrome (4,376) | ALT | 7 | |
| USA44 | Chronic Liver Disease Registry (742) | US/CT | 39.1 | |
| USA45 | Polycystic ovary syndrome (88) | US | 54.5 | |
| USA46 | Bariatric surgery patients (233) | Biopsy | 97 | 32.6 NASH, 31.3 fibrosis |
| Mexico47 | Metabolic syndrome (337) | US | 15.7 | |
| Mexico48 | Metabolic syndrome (198) | US | 82.9 | |
| Mexico49 | Polycystic ovary syndrome (197) | US | 62 | |
| Brazil50 | Bariatric surgery patients (146) | Biopsy | 76 | 41.1 NASH |
| Brazil51 | Bariatric surgery patients (112) | Biopsy | 99.1 | 55.7 NASH/ 21.6 fibrosis |
| Brazil52 | Diabetic patients (98) | Biopsy | 20 | 78 NASH/ 34 to 60 fibrosis 26.0 NASH/ 1.6 cirrhosis |
| Chile53 | Bariatric surgery patients (127) | Biopsy | 63 | 26.0 NASH/ 1.6 cirrhosis |
ALT: alanine aminotransferase. CT: computed tomography. US: ultrasound.
Studies of the prevalence of NAFLD in the Americas have mainly used US to diagnose the disease in large populations, whereas liver biopsy is mainly restricted to high-risk populations. The available data suggest that the prevalence of NAFLD is increasing in this continent (Tables 1 and 2).18,20
NAFLD is a complex condition that involves ethnic, genetic, and environmental factors that determine its development and progression. The prevalence of NAFLD increases with age and is highest in men aged 40-65 years. There is some debate about whether the prevalence is higher in men or women, but some studies have found that NAFLD and NASH are more common in men (Table 2). It is noteworthy that family members of NAFLD patients have a higher risk of developing NAFLD regardless of age and BMI.27
Risk FactorsEthnicityThe population in the Americas is a complex mix of human groups, especially in South America, one of the most diverse regions in the world. Ethnicity is an important predictor for chronic liver disease complications and has a strong influence on the response to treatment of chronic liver disease.44
NAFLD affects all ethnicities, although previous studies have shown that Hispanics have a higher prevalence of NAFLD than do African Americans or Caucasians and that Hispanics may be considered the ethnic group with the highest risk. This increased risk may reflect the higher rates of IR and visceral adiposity at equivalent BMI in Hispanics compared with other ethnic groups.44,54,55
A cohort study of 1,026 adults from several ethnic groups with biopsy-proven NAFLD studied the severity of the disease. Latinos with NASH were younger, performed less physical activity, had higher carbohydrate intake, and were more than six times more likely to have acanthosis nigricans compared with non-Latino whites with NASH who had Homeostasis Model of Assessment-IR as a significant risk factor and a higher prevalence of hypertension.56 These data suggest that NAFLD is more prevalent among Hispanics individuals and less prevalent among African Americans.19,30,44,54
GeneticsFamily and interethnic variation studies suggest that genetic factors are important risk determinants of the susceptibility to and severity of NAFLD (Table 3). Multiple polymorphisms in genes affecting lipid metabolism, cytokine regulation, fibrotic mediators, and oxidative stress may be associated with lipid content and with the development and progression of steatohepatitis and/or fibrosis.57
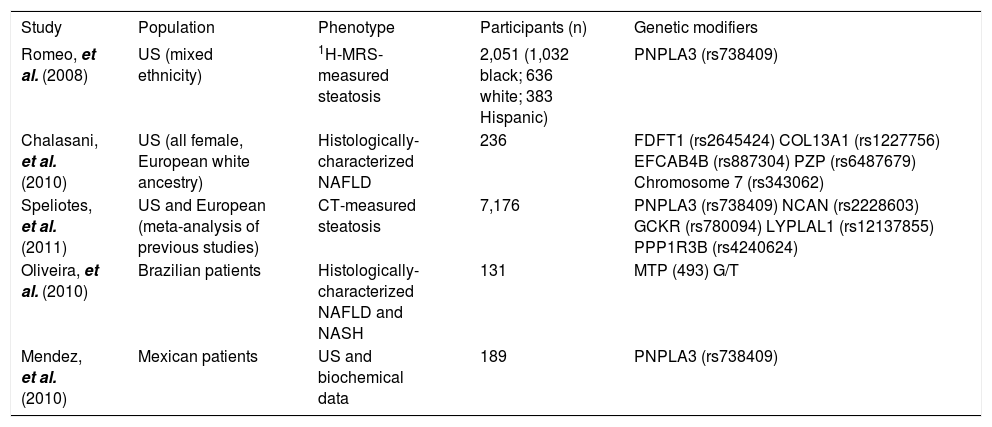
Studies in the Americas on genetic modifiers of NAFLD.
| Study | Population | Phenotype | Participants (n) | Genetic modifiers |
|---|---|---|---|---|
| Romeo, et al. (2008) | US (mixed ethnicity) | 1H-MRS-measured steatosis | 2,051 (1,032 black; 636 white; 383 Hispanic) | PNPLA3 (rs738409) |
| Chalasani, et al. (2010) | US (all female, European white ancestry) | Histologically-characterized NAFLD | 236 | FDFT1 (rs2645424) COL13A1 (rs1227756) EFCAB4B (rs887304) PZP (rs6487679) Chromosome 7 (rs343062) |
| Speliotes, et al. (2011) | US and European (meta-analysis of previous studies) | CT-measured steatosis | 7,176 | PNPLA3 (rs738409) NCAN (rs2228603) GCKR (rs780094) LYPLAL1 (rs12137855) PPP1R3B (rs4240624) |
| Oliveira, et al. (2010) | Brazilian patients | Histologically-characterized NAFLD and NASH | 131 | MTP (493) G/T |
| Mendez, et al. (2010) | Mexican patients | US and biochemical data | 189 | PNPLA3 (rs738409) |
Interestingly, inherited factors play a major role in the susceptibility to NASH. For example, the rs738409 C/G single-nucleotide polymorphism of patatin-like phospholipase domain-containing 3 (PNPLA3), which encodes the I148M protein variant, was identified by a genome-wide approach as a strong genetic determinant of liver fat content independently of body mass, dyslipidemia, and IR. This polymorphism also has been associated with NASH and progressive fibrosis in patients with steatosis.
Several studies have shown that polymorphism in the PNPLA3 gene predicts the extent of steatosis in NAFLD. For example, a recent meta-analysis estimated the strength of the effect of the I148 PNPLA3 variant on NAFLD and disease severity across different populations. This meta-analysis showed that rs738409 exerted a strong influence on liver fat accumulation and on the susceptibility to more aggressive disease.58
Other studies have investigated the association of polymorphisms of glutamate-cysteine ligase and microsomal triglyceride transfer protein (MTP) genes with NAFLD in Brazilian patients with biopsy-proven simple steatosis or NASH. The 493 G/T polymorphism in the MTP gene encodes the protein responsible for transferring triglycerides to nascent apolipoprotein B, and 129 C/T in the GCLC gene encodes the catalytic subunit of glutamate-cysteine ligase in the formation of glutathione. The presence of at least one T allele in the 129 C/T polymorphism of the GCLC gene was independently associated with NASH, whereas at least one G allele in the −493 G/T polymorphism of the MTP gene differed slightly between biopsy-proven NASH and simple steatosis. These two polymorphisms could represent an additional factor for consideration when evaluating the risk of NAFLD progression; however, further studies of larger populations are needed to confirm this notion.57
The Insulin Resistance Atherosclerosis Family Study (IRAS) quantified liver density with computed tomography and confirmed the association between a PNPLA3 variant and NAFLD in Hispanic Americans and African Americans. The rs738409 single-nucleotide polymorphism was strongly associated with reduced liver density in Hispanic Americans and African Americans. This study also suggested that PNPLA3 contributes to the differences in the risk of NAFLD between various ethnic groups.58
Recently, polymorphisms in the T-455C and C-482T APOC3 promoter region have been reported to predispose to dyslipidemia, insulin resistance, and NAFLD in Indian subjects. In Caucasian patients with NAFLD, APOC3 genotype is not associated with progressive liver damage, but the association with liver damage in Hispanics has not been evaluated.59
Several studies have been performed to investigate the role of heritability in NAFLD and its complications. In a familial aggregation study, Schwimmer et al. demonstrated that NAFLD is highly heritable. They concluded that hepatic steatosis was significantly more common in siblings (59%) and parents (78%) of children with NAFLD. These data suggest that familial factors are a major determinant of whether an individual develops NAFLD.60 Another study, involving eight members of eight kindreds with two or more patients with NASH and cryptogenic cirrhosis found that the presence of NASH with or without cryptogenic cirrhosis within these kindreds suggested a possible genetic risk.61
Chalasani, et al. in a pilot genome-wide association study of liver histology in non-Hispanic white women with NAFLD, analyzed clinical, laboratory, and histologic data. They found significantly associated genetic variants with features of hepatic histology in patients with NAFLD; however these findings should be validated in larger and more diverse cohorts.62
Environmental and lifestyle-related factorsEnvironmental and lifestyle-related factors such as a low physical activity level and a high-fat diet are associated with NAFLD development and are related to IR. The best predictors of NAFLD may be BMI and waist circumference.63 The former seems to have the strongest independent association with NAFLD. In one study, although waist circumference was a strong predictor of mortality in women, having NAFLD, smoking, and BMI were independent predictors of mortality in men.64
NAFLD is the hepatic manifestation of the MS. In most NAFLD patients, the disease is mainly associated with metabolic risk factors such as obesity (75-95%),4 T2DM and/or IR (70%), and dyslipidemia (50%).19 Up to two-thirds of patients with obesity and T2DM present with hepatic steatosis.65
The central obesity phenotype is associated with increased visceral fat content. The prevalence rates of NAFLD and NASH in the morbidly obese have been estimated to be as high as 91% and 37%, respectively.19,66 Patients with central obesity are characteristically insulin resistant and present more frequently with NAFLD than do patients with lower-body obesity.67
IR and T2DMIR and oxidative stress play an important role in NAFLD development and progression. The high concentration of insulin may cause failure to suppress fatty acid flux.68–70 The prevalence rates of NAFLD and NASH in diabetic patients have been estimated as 60-76% and 22%, respectively.65,71
IR is a major contributor to NAFLD, which is why prevention is important. A cross-sectional study in Chilean adults found that most people diagnosed with IR also had acanthosis nigricans and concluded that acanthosis nigricans may be an early marker of IR in this population.72 T2DM has been shown to be an independent risk factor for chronic liver disease in patients with NAFLD and is an independent risk factor for cirrhosis and HCC. Although most patients with simple fatty liver are not at risk of death, they are at increased risk of cardiovascular disease, and fatty liver disease doubles the risk of T2DM independently of the severity of liver injury.73
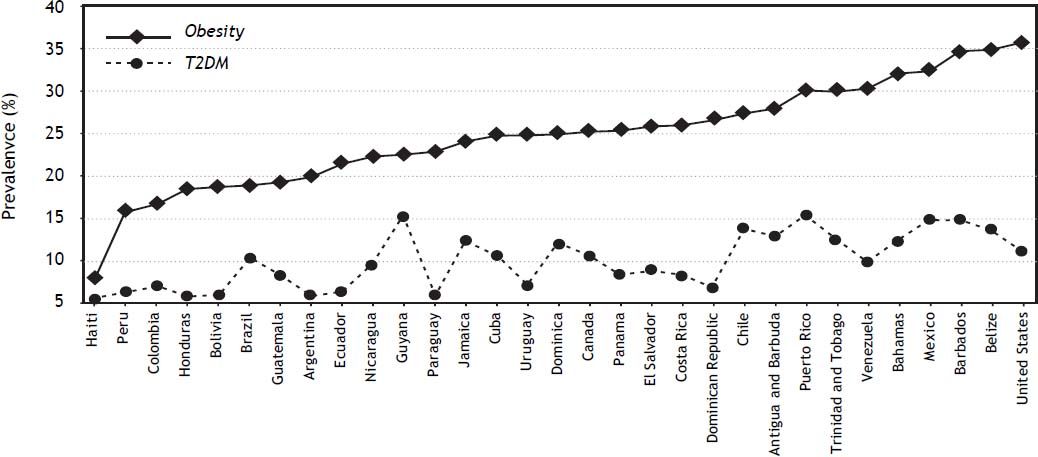
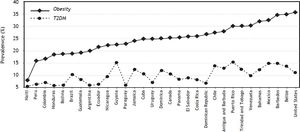
According to data from the International Diabetes Federation, North America and the Caribbean region have the second highest prevalence of diabetes: 10.7% of the adult population affected and an incidence of 1 in 10 adults. Specifically, Puerto Rico, Guyana, and Mexico have the highest prevalence of diabetes in the Americas (Figure 1). The USA has the most people −23.7 million− with diabetes followed by Mexico, Canada, and Haiti.74
The MSNAFLD is the main hepatic manifestation of the MS, and frequently coexists with obesity, dyslipidemia, and IR. The MS shares common mechanisms with NAFLD development, and both form the pathophysiological basis of IR. The MS is a common complex entity with a high worldwide prevalence that predicts, or is associated with, several diseases such as T2DM, cancer, and NAFLD. About 90% of NAFLD patients have more than one MS component, and one-third have the MS. There is no doubt that NAFLD and the MS are strongly related, but not all people with the MS will develop NAFLD, and vice versa.69,75,76
Yilmaz, et al.77 noted that 20-80% of NAFLD patients have at least one component of the MS.78–80 The diagnostic criteria used to define the MS influence the prevalence of both the MS and NAFLD. Thus, the prevalence of the MS in NAFLD cohorts may depend heavily on the definition of the syndrome.81
Studies from the Latin American Group for the Study of Metabolic Syndrome (GLESMO) tested waist circumference as a diagnostic tool for identifying people with visceral adiposity in Hispanics from the Latin American region. The Latin American Diabetes Association suggested the use of the International Diabetes Federation definition with new Latin American criteria. However, for epidemiological studies, it is advisable to also use the Adult Treatment Panel (ATP) III criteria.82 Although there are insufficient data from all Latin American countries, the available data show that the prevalence of the MS is consistent between countries and depends on the definition used, age ranges, the proportion of men and women included in the studies, and the type of population (urban, rural, aboriginal) (Table 4).82
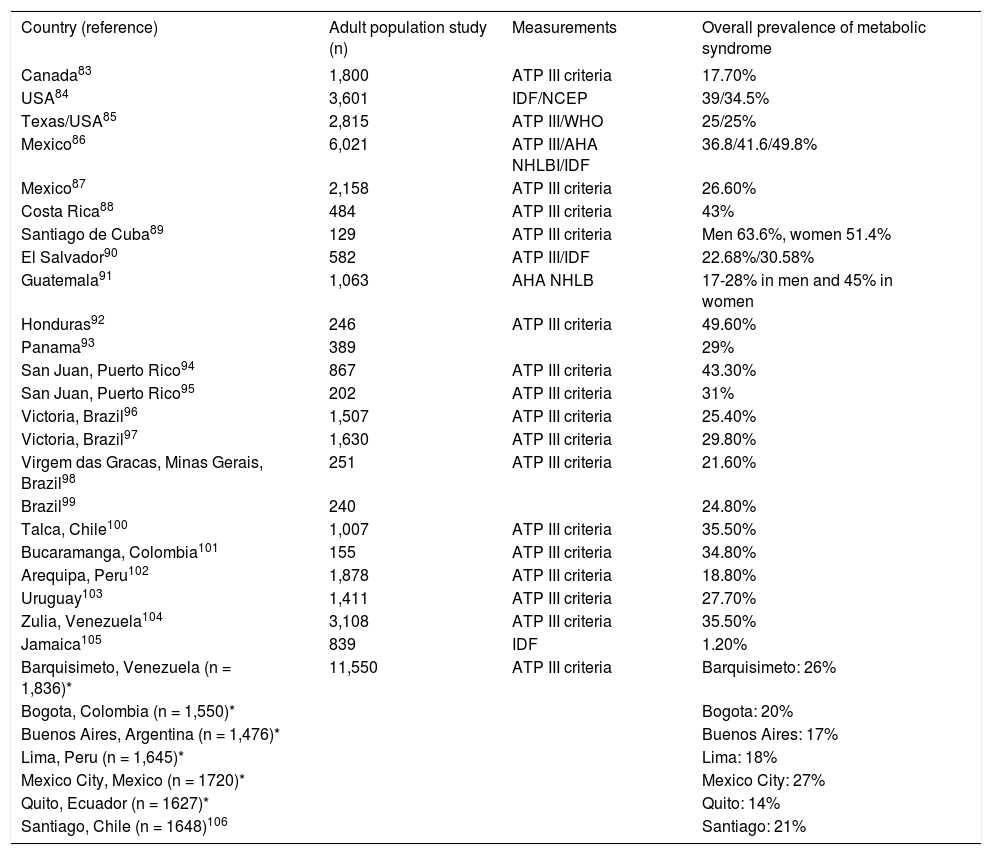
Prevalence of metabolic syndrome in the Americas.
| Country (reference) | Adult population study (n) | Measurements | Overall prevalence of metabolic syndrome |
|---|---|---|---|
| Canada83 | 1,800 | ATP III criteria | 17.70% |
| USA84 | 3,601 | IDF/NCEP | 39/34.5% |
| Texas/USA85 | 2,815 | ATP III/WHO | 25/25% |
| Mexico86 | 6,021 | ATP III/AHA NHLBI/IDF | 36.8/41.6/49.8% |
| Mexico87 | 2,158 | ATP III criteria | 26.60% |
| Costa Rica88 | 484 | ATP III criteria | 43% |
| Santiago de Cuba89 | 129 | ATP III criteria | Men 63.6%, women 51.4% |
| El Salvador90 | 582 | ATP III/IDF | 22.68%/30.58% |
| Guatemala91 | 1,063 | AHA NHLB | 17-28% in men and 45% in women |
| Honduras92 | 246 | ATP III criteria | 49.60% |
| Panama93 | 389 | 29% | |
| San Juan, Puerto Rico94 | 867 | ATP III criteria | 43.30% |
| San Juan, Puerto Rico95 | 202 | ATP III criteria | 31% |
| Victoria, Brazil96 | 1,507 | ATP III criteria | 25.40% |
| Victoria, Brazil97 | 1,630 | ATP III criteria | 29.80% |
| Virgem das Gracas, Minas Gerais, Brazil98 | 251 | ATP III criteria | 21.60% |
| Brazil99 | 240 | 24.80% | |
| Talca, Chile100 | 1,007 | ATP III criteria | 35.50% |
| Bucaramanga, Colombia101 | 155 | ATP III criteria | 34.80% |
| Arequipa, Peru102 | 1,878 | ATP III criteria | 18.80% |
| Uruguay103 | 1,411 | ATP III criteria | 27.70% |
| Zulia, Venezuela104 | 3,108 | ATP III criteria | 35.50% |
| Jamaica105 | 839 | IDF | 1.20% |
| Barquisimeto, Venezuela (n = 1,836)* | 11,550 | ATP III criteria | Barquisimeto: 26% |
| Bogota, Colombia (n = 1,550)* | Bogota: 20% | ||
| Buenos Aires, Argentina (n = 1,476)* | Buenos Aires: 17% | ||
| Lima, Peru (n = 1,645)* | Lima: 18% | ||
| Mexico City, Mexico (n = 1720)* | Mexico City: 27% | ||
| Quito, Ecuador (n = 1627)* | Quito: 14% | ||
| Santiago, Chile (n = 1648)106 | Santiago: 21% |
The general prevalence (weighted mean) of the MS in Latin American countries is 24.9%. The MS occurs slightly more frequently in women (25.3%) than in men (23.2%), and the prevalence is highest in people older than 50 years. The most frequent components of the MS are a low blood high-density lipoprotein cholesterol level (62.9%) and abdominal obesity (45.8%).
Although several studies have reported the prevalence of the MS in Latin America, in most studies, the MS was a secondary objective of the study. Thus, the prevalence of this syndrome may not reflect the real prevalence among the general population.
ObesityThe extent of obesity correlates strongly with NAFLD prevalence and severity. Recent data show that the prevalence of obesity in the Americas (23.5% in males and 29.7% in females) is the highest in the world. The Americas have the highest prevalence of obesity in adults aged 20 years or more.107 The trends between 1980 and 2008 indicated a rapid increase in the prevalence of obesity, even in countries with currently low rates.
The prevalence of obesity differs between ethnic groups in the Americas. For example, non-Hispanic Afro-Americans have the highest age-adjusted rates of obesity (49.5%) compared with Mexican Americans (40.4%), Hispanics (39.1%), and non-Hispanic whites (34.3%).108
The Pan American Health Organization reports that in Argentina, Colombia, Peru, Paraguay, and Uruguay, more than 15% of the entire population is obese. National comparative studies have found that the prevalence of obesity in the adult population is > 20% in 17 of 20 Latin American countries.21 In all South American countries except for Brazil, Argentina, Colombia, and Bolivia, > 50% of the population is overweight.21 In Bolivia, Chile, Ecuador, and Peru, 11-15% of the adolescent population is obese. In this age group, obesity is more prevalent in urban areas and in areas with low socioeconomic status. A summary of the data on the prevalence of obesity in America (Figure 1) shows that the USA, Mexico, Barbados, and Belize have the highest prevalence of obesity. Using the available data, several projections have estimated that by 2030, there will be 65 million more obese adults in the USA.109
Prevalence of NAFLD in the Americas estimated from obesity ratesThe largest increases in the prevalence of the MS have occurred in the USA, Mexico, Costa Rica, Puerto Rico, Chile, and Venezuela. Using information from the Cardiovascular Risk Factor Multiple Evaluation in Latin America (CARMELA)106 study, we confirmed that Chile, Mexico, and Venezuela are countries with a high prevalence of MS in Latin America.
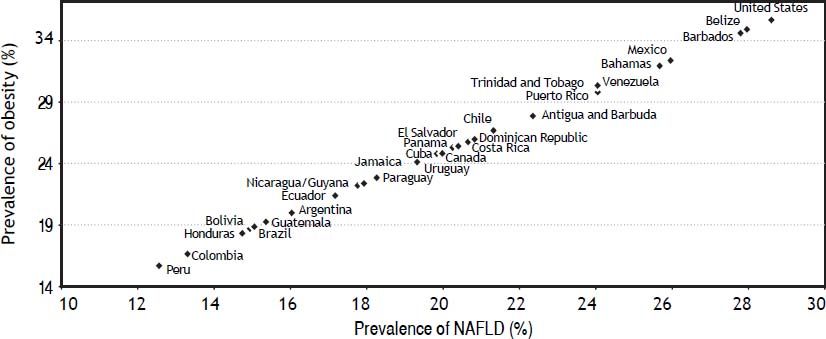
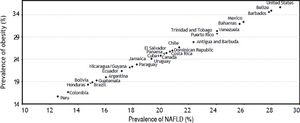
Despite the relative lack of studies on the epidemiology of NAFLD in emerging regions such as Latin America, we believe that the prevalence of obesity-associated diseases such as NAFLD in the Americas can be estimated from the prevalence rates of obesity. Assuming that about 80% of obese patients might develop NAFLD, we predicted the prevalence for different countries, as shown in figure 2 and discussed below.
Correlation between the prevalence of Obesity and NAFLD in the Americas. The graph was built with data from the prevalence of obesity for each country; NAFLD prevalence was estimated assuming that about 80% of obese patients might develop NAFLD4 in the Americas Countries.
Based on the prevalence of obesity, the prevalence rates of NAFLD are estimated as 26% in Mexico, 29% in the USA, 15-20% in Central America, and 28% in Belize and Barbados, which have high obesity prevalence. In South America, the prevalence of NAFLD have been estimated as 24% in Venezuela and Chile, 20% in Uruguay, 18% in Guyana, Paraguay, and Ecuador, and ≤ 16% in the other countries.
In summary, we found that the prevalence of obesity and NAFLD in the Americas are highest in the USA, Belize, Barbados, and Mexico.
Knowing the prevalence of NAFLD in the Americas will help establish appropriate preventive measures because the early recognition and treatment of this disease are critical given its important role in the development and progression of cardiovascular disease.
The management of risk factors is extremely important for limiting the burden of NAFLD. The present review brings us closer to an understanding of the prevalence of NAFLD in the Americas. However, it is not possible to know the full scope of the problem, partly because data from some countries are not available and because methodological differences between studies limit a combined analysis of their results.
ConclusionThe prevalence of NAFLD in the Americas is highest in the USA, Belize, Barbados, and Mexico. the increasing prevalence of NAFLD in American countries is likely to be linked to an increased prevalence in obesity; this will present a challenge in the coming years in terms of public health costs. Efficient and well-planned preventive strategies based on lifestyle modification and nutritional interventions focused mainly on the pediatric population should be implemented to reduce the disease burden. This would likely reduce the impact on health-service costs related to chronic liver disease in these nations.
Abbreviations- •
1H MRS: proton magnetic spectroscopy.
- •
BMI: body mass index.
- •
CARMELA: Cardiovascular Risk Factor Multiple Evaluation in Latin America.
- •
HCC: hepatocellular carcinoma.
- •
IR: insulin resistance.
- •
MS: metabolic syndrome.
- •
MTP: microsomal triglyceride transfer protein.
- •
NAFLD: nonalcoholic fatty liver disease.
- •
NASH: nonalcoholic steatohepatitis.
- •
PNPLA3: patatin-like phospholipase domaincontaining 3 gene.
- •
T2DM: diabetes mellitus.
- •
US: ultrasound.
- •
USA: United States of America.