Utilization of palliative care services in patients dying of end-stage liver disease (ESLD) is understudied. We performed a retrospective review of palliative care services among patients with ESLD unsuitable for liver transplantation (LT) at a tertiary care center.
Material and MethodsDeceased ESLD patients considered unsuitable for LT from 2007-2012 were identified. Patients were excluded if they received a transplant, had an incomplete workup, were lost to follow up or whose condition improved so LT was not needed. Of the 1,175 patients reviewed, 116 met inclusion criteria.
ResultsForty patients (34.4%) received an inpatient palliative care (PC) consultation and forty-one patients (35.3%) were referred directly to hospice. Thirty-three patients (28.4%) transitioned to comfort measures without PC consultation (median survival < 1 day). The median interval between LT denial and PC consultation or hospice was 28 days. Median survival after PC consult or hospice referral was 15 days. In conclusion, in a single center retrospective review of ESLD patients, palliative care services, when utilized, were for care at the very end of life. Without consultation, aggressive interventions continued until hours before death. We propose that ESLD patients could benefit from PC consultation at time of LT evaluation or based on MELD scores.
Patients with cirrhosis who develop complications, such as jaundice, ascites, hepatic encephalopathy, and/or gastrointestinal hemorrhage, are considered to have end-stage liver disease (ESLD). ESLD is a significant cause of morbidity and mortality. It is the seventh leading cause of death in the U.S. with a 2-year survival of less than 50% after the development of ascites or encephalopathy. ESLD patients frequently are not eligible or die waiting for liver transplantation (LT) and do so with significant symptom burden and family distress.1-3 This population requires expert medical management and are often hospitalized.4 Hitherto, medical professionals treating ESLD patients have underutilized palliative care and expert opinion has called for more frequent and earlier involvement of palliative care services.4-6
To date, there are scant data on the utilization of palliative care services in patients with ESLD. A recent retrospective study conducted at the University of Alberta, Canada, by Poonja, et al. showed that patients with ESLD, who were not LT candidates, had high a symptom burden and low rates of documented advance care planning.7 Despite this and poor patient survival (median survival of 52 days from denial of LT to death), these patients were infrequently referred to receive palliative care services. In addition, despite removal from the LT waiting list, a significant number of patients in this study went on to be hospitalized, only to receive potentially unwanted care in the Intensive Care Unit with invasive treatments, such as renal replacement therapy.8
We postulated that the ESLD patient population was particularly suited to, and would derive real benefit from, early integration of specialty level palliative care services. The purpose of the present study was to characterize how our institution uses palliative services (inpatient consultation and hospice) in the care of liver patients with poor prognosis who were denied liver transplantation.
Material and MethodsWe performed a retrospective study to evaluate utilization of palliative care services among all patients with ESLD who were evaluated for liver transplantation (LT) at the University of Wisconsin Hospitals and Clinics (UWHC), found to be ineligible for LT and at risk for a liver-related death. We reviewed liver transplant selection committee meeting minutes at UWHC from 2007-2012 and compiled a database that included 1,175 patients. Patients who were living at the time of chart review were excluded to ensure a complete medical record. Other exclusion criteria included patients who remained on the transplant wait list, received a transplant at any time, had an incomplete transplant workup, were lost to follow up, or whose condition improved so they did not require transplantation. Patients with prior transplants were excluded to eliminate potential biases with regard to re-transplantation and palliative care involvement. One hundred sixteen patients met all criteria and were included for chart abstraction. Figure 1 describes the process of determining the study cohort based on our exclusion criteria. The protocol was approved by the University of Wisconsin Institutional Review Board. Although we used the medical records of human subjects in our study, their consent was not needed per the Institutional Review Board.
We abstracted multiple clinical parameters at time of presentation including gender, MELD score, age, etiology of liver disease, prior listing status, cause of death and events between LT denial and palliative consult. We recorded relevant dates including LT denial, palliative consult, hospice enrollment and death. Time intervals (in days) between these events were calculated.
UWHC has an inpatient palliative care consult service staffed by a physician, nurse, and social worker and has an active fellowship training program and is available 24 h a day. UW did not have an ambulatory PC clinic. Palliative care consultations have a number of discrete objectives. They involve a discussion of medical issues and assisting patients to identify personal goals for end-of-life care. They also involve assessment and management of physical symptoms, assessment and management of psychoso-cial, spiritual and practical needs and lastly, assessment of discharge planning issues.9 The end result of PC consultations may entail no changes in care, amendment of medical therapies, changes in code status, transfer to an outpatient or inpatient hospice unit or a number of other interventions. PC consultation may be suggested by any member of the care team, consultants, the patient or their relatives. At the time of this study, ESLD patients were admitted to general medicine, hospitalist or intensive care services. Ultimately, PC consult orders are placed at the discretion of the attending physician on these services, respectively. There were no specific protocols regarding the circumstances for, or the timing of, PC consultation.
ResultsOne hundred sixteen patients met the entry criteria. At time of transplant denial, median age was 58.5 years and 44 patients (37.9%) had been on the liver transplant waitlist at some point. Seventy-five patients (65%) were male and the median MELD score was 22.5. Additional demographic information for the cohort is detailed in table 1.
Patient demographics and diagnoses.
| Median age in years Median MELD | 58.5 (range 20-78) 22.5 |
|---|---|
| Gender | 75 male (65%) |
| Waitlisted at some point | 44 (37.9%) |
| Advance directive on file | 88 (75.9%) |
| Hospitalized after denial | 33 (28.4%) |
| ICU stays after denial | 9 (8%) |
| Etiology of liver disease | Patients, n (Percentage of cohort) |
| ALD | 30 (25.8%) |
| HCV | 10 (8.6%) |
| ALD + HCV | 20 (17.2%) |
| NASH | 22 (19%) |
| HCC+HCV | 15 (12.9%) |
| HCC + ALD | 7 (6%) |
| Primary sclerosing cholangitis | 5 (4.3%) |
| ALD + NASH | 4 (3.4%) |
| Autoimmune hepatitis | 1 (0 9%) |
| Primary biliary cirrhosis | 1 (0.9%) |
| Acetaminophen | 1 (0.9%) |
| Reason for denial | Patients, n |
| or removal from waitlist | (Percentage of cohort) |
| Sepsis | 25 (21.6%) |
| Multi-organ system failure | 14 (12.1%) |
| CAD/PAD | 14 (12.1%) |
| Alcohol abuse | 13 (11.2%) |
| HCC beyond Milan criteria | 12 (10.3%) |
| Psychosocial issues | 7 (6%) |
| Patient choice | 5 (4.3%) |
| Extra-hepatic malignancy | 5 (4.3%) |
| Obesity | 5 (4.3%) |
| Other substance abuse | 4 (3.5%) |
| Advanced age | 4 (3.5%) |
| Other substance abuse | 4 (3.5%) |
| Portal vein thrombosis | 3 (2.6%) |
| CVA | 3 (2.6%) |
| NPH | 1 (0.9%) |
| Cervical spine fracture | 1 (0.9%) |
ALD: alcoholic liver disease. HCV: hepatitis C virus. NASH: non-alcoholic steatohepatitis. HCC: hepatocellular carcinoma. CAD: coronary artery disease. PAD: peripheral artery disease. CVA: cerebrovascular accident. NPH: normal pressure hydrocephalus.
A typical PC consult was inpatient and specifically for a transition to end of life care. Forty patients (34.4%) received at least one inpatient palliative care consultation. Among those 40 patients, 9 were transferred to the inpatient palliative care service and 15 were referred to hospice for care at the end of life. The remaining 16 patients evaluated by the PC consult team continued to receive their assistance without hospice or transfer to their service. Forty-one patients (35.3 %) were referred directly to hospice as outpatients. Including those who had a palliative consultation, a total of 65 patients (56%) received inpatient or residential hospice care with an average length of stay (LOS) of 20 days. There was a considerable difference in hospice LOS between patients seen by the inpatient palliative care team (mean 12 days) vs. patients who were referred directly to hospice (mean 26.5 days). This likely reflects a higher degree of illness among inpatients with ESLD relative to outpatients. Thirty-three patients (28.4%) transitioned to comfort measures without palliative consultation. Figure 2 summarizes the palliative services rendered to the study cohort.
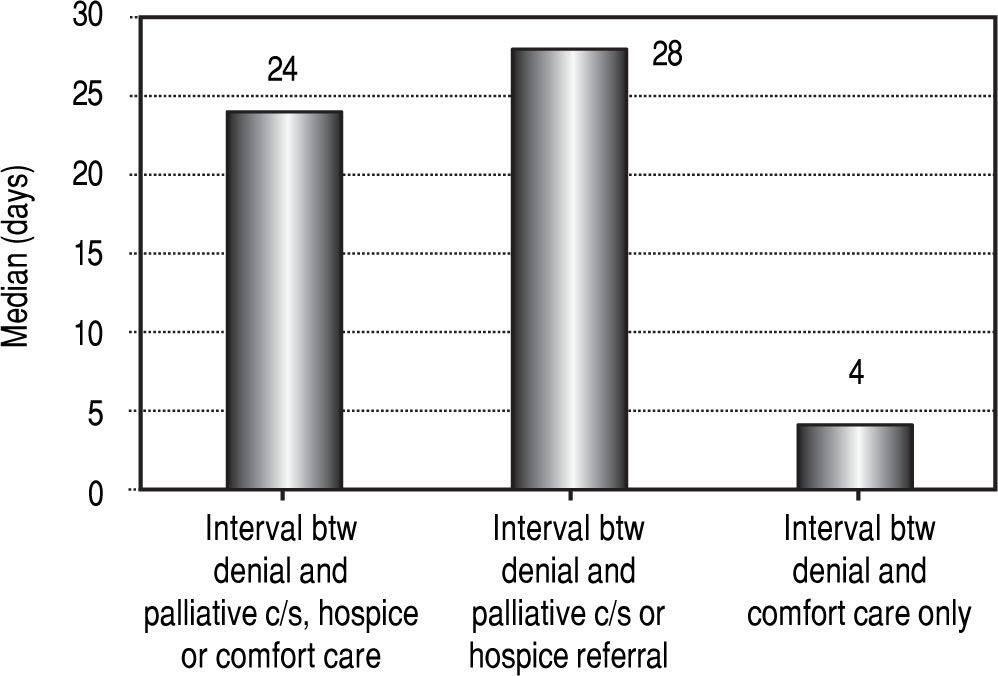
Timing of palliative interventions; Patient factorsFor the entire cohort, the median interval between denial of LT listing and palliative care consultation, hospice referral or initiation of comfort measures was 24 days. We then performed separate analyses on the patients who had palliative care consultation or hospice referral and those who had comfort care only without specialist involvement. The median interval between denial of LT listing and referral to palliative care / hospice group was 28 days, while the median interval between denial and comfort care only was 4 days. Figure 3 summarizes these time intervals. The palliative care / hospice group had an average MELD score of 20.5 and the comfort care only group had an average MELD of 30.3
Median intervals in days between denial of liver transplantation and palliative interventions. Column 1 represents the entire cohort (24 days), column 2 represents patients who received a palliative consult or hospice referral (28 days) and column 3 represents patients who received comfort measures only (4 days).
Median survival after denial of LT listing for the entire cohort was 28 days, excluding 18 patients (15.5%) with unknown date of death. Median survival after palliative care consult or direct hospice referral was 15 days, compared to 0 days for patients who received comfort care only. There were no clear trends toward longer or shorter survival times during the study period. Figure 4 illustrates the median duration of survival for the entire cohort, palliative consult / hospice group and comfort care only group. Median durations for survival following palliative consult / hospice referral or comfort care only, respectively, are shown as well. Most notably, among the 81 patients who had a palliative consultation or were referred to hospice, 27 (33%) died within one week of palliative involvement. Half of the comfort care only group (16/32) died on the day that comfort measures were initiated and the remainder died within the following 2 days.
Median survival in days following denial of liver transplantation. Column 1 represents the entire cohort (28 days), column 2 represents patients who received a palliative consult or hospice referral (47 days) and column 3 represents patients who received comfort measures only (4 days). Column 4 represents median survival after palliative consult or hospice referral (15 days) and column 5 represents median survival after instituting comfort measures (0 days).
Advance directives were on file for 88 patients (75.9%) in the form of naming a healthcare power of attorney, having a living will or clearly defining their code status. Thirty-three patients (28.4%) were hospitalized following denial of listing, excluding admissions for palliative treatments such as HCC ablation. The mean number of hospital stays was 0.73 among the entire cohort and 2.66 among those with one or more stays. The mean inpatient length of stay LOS was 4 days among entire cohort and 15 days among patients with one or more stays. We did not see a trend toward lower or higher rates of admissions during the study period. After denial of listing or removal from the list, 4 patients were initiated on renal replacement therapy, 7 required mechanical ventilation and 3 underwent upper endoscopy with banding of esophageal varices. Twenty-four patients (20.6%) were in the Intensive Care Unit at the time of death, though most patients were already there when denied listing or removed from the waitlist. Among those who were denied listing beforehand, 9 patients (8%) required admissions to the intensive care unit with an average LOS of 7.3 days.
ConclusionsPalliative care is both a medical specialty and a philosophy of care provided to people and families facing a serious illness, often focusing on the relief of symptoms while seeking to best align treatments with patient preferences. Palliative care is appropriate for patients of any age and at any stage of a serious illness, whether or not the illness is curable. Philosophically, palliative care may be provided by any clinician or by an expert who received additional training from their respective discipline (i.e. physician, nursing, social work, or spiritual care). While the evidence base is most robust in the care of patients with cancer, it is no longer confined to patients with cancer or to patients facing imminent death. It values the autonomy of the patient and has been aptly summarized by Boyd, et al. as ‘living and dying well’.5 Palliative care relies on dialogue with, and listening to, the patient and requires candor and flexibility on the part of the medical team and caregivers. This has led to the development of palliative care as a medical subspecialty, and palliative care is often directed by specialists who work in conjunction with the patient's primary management team.
When palliative care is integrated with standard care at the time of diagnosis of a severe illness, several patient and system-related factors are improved including quality of life, satisfaction, symptom burden, advanced care planning documentation, while emergency room utilization and costs of care decline.9-13 In addition to these benefits, some studies have shown a significant survival advantage among the early palliative care cohort.13 Importantly, none of the randomized trials of integrated palliative care have shown harm or detriment. Studies in which palliative care providers are involved at the end of life, rather than at the time of diagnosis, have failed to demonstrate the same magnitude of benefit.14-16 It appears that this type of care is more effective when the providers have the opportunity to work with patients to help them prepare before they are gravely ill and no longer able to participate fully due to medical illness or psychological distress.
Making the transition from potentially life-saving treatment, such as a LT, to discussing end-of-life care is particularly challenging. One study showed that consideration for LT was associated with a 7% lower-quality end-of-life care score, due to lack of timely discussions and advance care planning.8 This makes patients with ESLD prime candidates for the concurrent care model in which palliative care is integrated with active medical treatment. The advantage of such a model is that it offers the patient ongoing care and support throughout their disease course, regardless of their transplant status. Medici, et al. evaluated this concept through a prospective study in which 157 patients were admitted to hospice, including 15 patients simultaneously listed for liver transplantation.17 In their population the mean MELD score was 21, the mean length of hospice stay was 38 days and 6 patients underwent liver transplantation during the study. There was a significant correlation between hospice length of stay and MELD score. Their findings suggest that MELD score, performance status, weight loss and the presence of a hepatic malignancy are useful metrics for initiating palliative care services among patients with serious liver disease.17
Our study illustrates a number of important points regarding the care of patients with ESLD in our center. In contrast to Poonja, et al. mentioned above, who found that only 11% of patients were referred for palliative care services, 70% of patients in our cohort (81/116) had a palliative care consultation or were referred directly to hospice. Twenty-eight percent of patients in their cohort had advanced care directives and 48% were admitted to the ICU, compared to 75% and 8% in our cohort, respectively. We recognize that these data may overestimate the use of palliative care in ESLD at UWHC since our study only included patients who had died and consequently had more pressing needs for palliative care than patients who remain alive. Furthermore, our cohort was limited to a single center in which access to palliative care services may be more readily available than in other hospital-based set-tings.18
We were unable to perform a meaningful cost analysis in our study since there was not a control group for comparison. In 2008, Morrison, et al. published a landmark study regarding cost savings associated with PC consult programs among 8 hospitals in the US from 2002-2004. They matched 2,630 PC patients who were discharged alive to 18,427 usual care patients and matched 2,278 PC patients who died to 2,124 usual care patients. PC patients who were discharged alive had an adjusted net savings of $1696 in direct costs per admission (p = 0.004) and $279 in direct costs per day (p < 0.001). Those PC patients who died before discharge had an adjusted net savings of $4908 in direct costs per admission (p = 0.003) and $374 in direct costs per day (p < 0.001). Direct costs are those that can be directly attributed to procedures, medications or services and costs were converted into 2004 US dollars.19 Although these results cannot be generalized to patients with ESLD and costs have changed since 2004, our study presents a situation where timely PC consultation has the potential to improve patient satisfaction and quality of life, while reducing healthcare expenditures.
Despite a high percentage of patients receiving palliative interventions in our study, these services were initiated very late in the disease process. Fifty-five percent of patients in our study received inpatient or residential hospice care and the median survival after transfer to hospice was only 15 days. Even within that group of patients, those who were referred directly to hospice had a longer survival than those who were seen by the inpatient palliative medicine team. This presumably reflects a higher level of illness among inpatients with ESLD. One-third of the patients in our cohort who received palliative care services had their first consultation within one week of death. Although this is a disappointing figure, survival after palliative consultation / hospice referral was considerably longer than survival after initiation of comfort measures only. Strikingly, half of the comfort care only group died within hours of initiating comfort measures and the remainder died within 2 days. The comfort care only group also had higher MELD scores at time of LT denial than the palliative care / hospice group (30.3 vs. 20.5, respectively) despite having comparable intervals between denial and palliative consult / hospice or initiation of comfort care measures. Therefore, patients who had the most severe illness and, potentially, could have the greatest benefit from palliative consultation, were often not evaluated by the PC team at all. Similar to the findings of Medici and colleagues, this highlights a meaningful opportunity for triggering palliative interventions on the basis of MELD score. While MELD scores of 15 estimate a 3 month mortality of 13%, this risk increases to 25% with MELD scores of 20.20 If palliative care is not initiated at time of LT evaluation, MELD scores ≥ 20 may be a useful threshold.
Our results are limited by the retrospective nature of the study, which predisposes to selection bias. Another drawback to our retrospective design is the inability to make direct comparisons of the palliative care / hospice group and the comfort care only group. Since patients were not randomized into these groups, we cannot control for confounders and cannot evaluate the impact of palliative care / hospice referral from a statistical standpoint. Additionally, we were unable to gather information on patient satisfaction, symptom burden or quality of life among patients and their families in the study cohort. Although a majority of patients (75.9%) had some form of advanced directive filed in the medical chart, it remains unclear how closely their care mirrored those wishes.
We interpret our data as indicating that whereas there is a high awareness of palliative care among managing physicians in our center, palliative care consultation is primarily obtained for assistance in end of life management. It appears that we see palliative care as useful for ‘dying well’ but we have not adopted palliative services as a model for assisting the terminally ill liver patient in living well during their final months or years.
In conclusion, our cohort was an extremely vulnerable population whose only chance for long-term survival, liver transplantation, was no longer available. Palliative care was instituted after removal from the LT list or denial of listing in the majority of our patients, but its introduction was generally delayed until patients were extremely ill and in the final few days of life. Readmission to the hospital and other significant interventions were common after LT had been denied. We suggest that prospective studies be conducted to investigate how early integration of palliative care might alter the experience of living or dying in patients facing all the challenges that come with ESLD including survival, transplant outcomes, quality of life, and symptom burden. We propose that the liver transplant evaluation include a palliative care consultation with goals of characterizing the patient's illness understanding, providing support for this high risk decision, completing an advance care plan, using decision support tools such as Best Case/Worst Case to explore a hypothetical negative outcome, and addressing unmet symptom management needs.21,22 We anticipate the concurrent care model, which incorporates palliative services into the LT evaluation, will improve patient satisfaction and quality of life without negatively impacting their survival.
Abbreviations- •
ESLD: End-Stage Liver Disease.
- •
LOS: Length of stay.
- •
LT: Liver transplantation.
- •
MELD: Model of End-stage Liver Disease.
- •
PC: Palliative Care.
- •
UWHC: University of Wisconsin Hospitals and Clinics
There are no conflicts of interest among the authors.
Funding SourcesDr. Campbell is supported by the Cambia Health Foundation's Sojourns Scholars Leadership Program. The funding source had no role in the design, preparation, review, or approval of the manuscript for publication.
AcknowledgementsNone.