Background. Bile leakage testing may help to detect and reduce the incidence of biliary leakage after hepatic resection. This review was performed to investigate the value of the White-test in identifying intraoperative biliary leakage and avoiding postoperative leakage.
Material and methods. A systematic review and meta-analysis was performed. Two researchers performed literature research. Primary outcome measure was the incidence of post-hepatectomy biliary leakage; secondary outcome measure was the ability of detecting intraoperative biliary leakage with the help of the White-test.
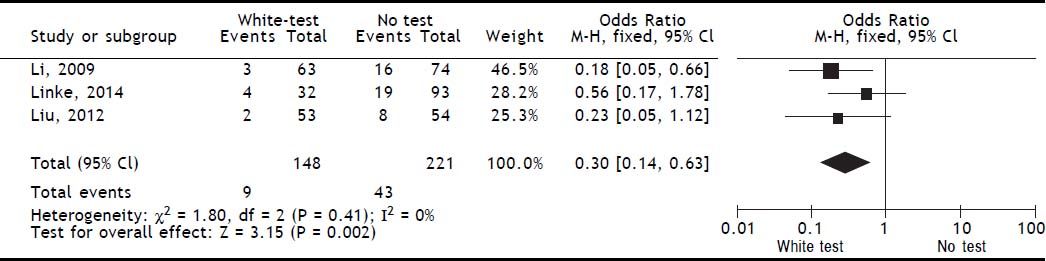
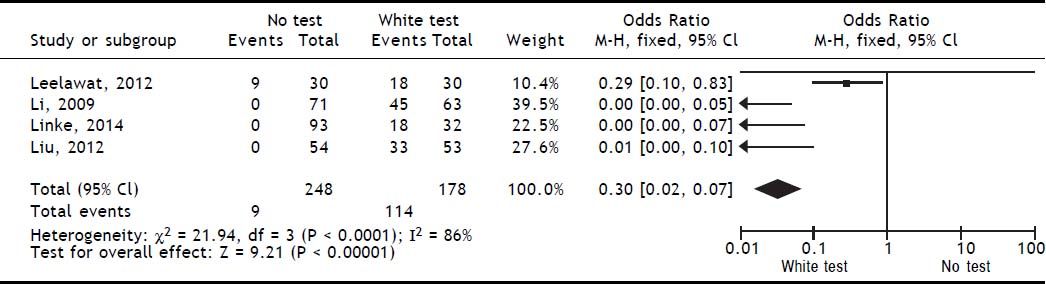
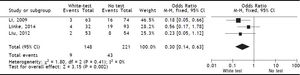
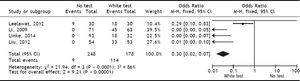
Results. A total of 4 publications (including original data from our center) were included in the analysis. Evidence levels of the included studies had medium quality of 2b (individual cohort studies including low quality randomized controlled trials). Use of the White-test led to a significant reduction of post-operative biliary leakage [OR: 0.3 (95% CI: 0.14, 0.63), p = 0.002] and led to a significant higher intraoperative detection of biliary leakages [OR: 0.03 (95%CI: 0.02, 0.07), p < 0.00001].
Conclusion. Existing evidence implicates the use of the White-test after hepatic resection to identify bile leaks intraoperatively and thus reduce incidence of post-operative biliary leakage. Nonetheless, there is a requirement for a high-quality randomized controlled trial with adequately powered sample-size to confirm findings from the above described studies and further increase evidence in this field.
Hepatic surgery is one of the most challenging surgical procedures due to its anatomical and pathophysiologic varieties and features.1 Once a resection has been performed, various problems can complicate the post-operative course. One of the major contributors to morbidity is the occurrence of biliary leakages which is present in up to 30% of patients after hepatic surgical procedures.2–5 Intraoperative bile leakage testing is one of the methods to prevent or at least reduce the incidence of postoperatively occurring bile leaks. The White-test seems to be the most promising test. In brief, a cannula is inserted in either the cystic duct after cholecystectomy or the common bile duct or right or left hepatic duct (depending upon the procedure). A fatty emulsion (e.g. 5% fat content parenteral nutrition supplement) is injected with gentle pressure. In case of biliary leakage extravasation of fatty emulsion can be detected and the leak oversewn accordingly. The test can be repeated without contamination of the resection surface at the surgeon’s choice, because the fatty emulsion can easily be washed away with saline without leaving any color behind. Various reports implicate the usefulness of the White-Test in the detection and reduction of biliary leakage.6–9 Moreover, a recent meta-analysis investigating various intraoperative biliary leakage testing by Wang, et al. revealed superiority of the White-test vs. other tests like application of methylene-blue and others.10 We thus performed a systematic review with validation of the existing evidence for the White-test vs. no intraoperative testing. The investigation of the potentially best intraoperative biliary leakage test should serve as hypothesis driving analysis for high-quality randomized controlled trials in the future.
Material and MethodsSystematic literature searchDatabases (Cochrane, PICO-search, pubmed.com, EMBASE and google.scholar) were screened for studies investigating the White-Test during hepatic resection with the search terms “White-Test”, “biliary leakage”, “bile leakage”, “bile leakage test”, and “biliary leakage test”. The search was performed for all years available ending in February 2014 to include the most recent manuscripts. Only publications in German and English language investigating the White-test (alternatively referred to as fatty-emulsion test) were included in the review and meta-analysis. Two authors (RL and AAS) reviewed the manuscripts for suitability (duplicate review) and only if both gave a positive vote, the manuscript was included in the analysis. In case of conflicts were observed, a third author (FU) was asked for suitability and the decision of in- or exclusion based on a simple majority.
Inclusion and exclusion criteriaThe primary aim was to include randomized controlled trials, large cohort-studies investigating the White-test vs. no intraoperative biliary leakage test or at least well-matched case-controlled studies into this systematic review and meta-analysis. Additionally, our original data of a cohort-study with 125 patients were included in this analysis. Patients should undergo elective hepatic surgery. All trials investigating biliary-leakage tests other than the White-test (e.g. saline-application, or methyleneblue application or indocyanine-green application) were excluded from the analysis.
Primary and secondary outcome measureA primary comparison was made for the incidence of post-operative biliary leakages after White-testing vs. no specific testing during hepatic surgery. Secondary outcome measure was a positive finding of intraoperative biliary leakages after White-testing vs. no specific testing.
Assessment of quality of included studiesThe analyzed manuscripts did not undergo specific quality assessment with Jadad-scoring for randomized controlled trials, or the Newcastle-Ottawa score for non-randomized trials due to a broad noncompliance and hazard of unregistered clinical trials to these statements.11–13 Instead we performed a critical review of evidence levels, which led to considerably lower evidence levels and classified evidence in the particular included trials in accordance with the definition of evidence of the Centre of Evidence in Medicine as outlined in table 1.14 Moreover the methodological quality of each included study was assessed using the questions outlined in table 2.
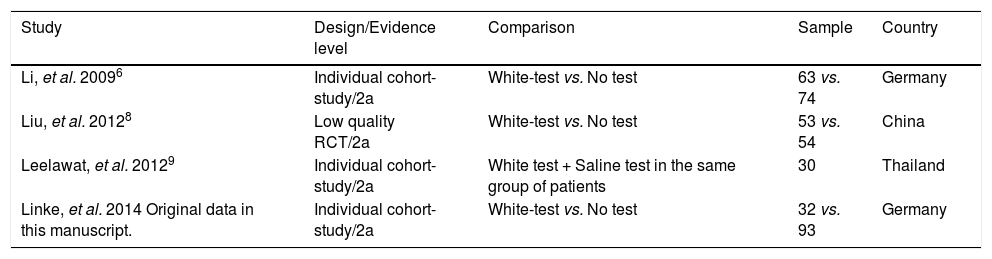
Basic data on included studies.
| Study | Design/Evidence level | Comparison | Sample | Country |
|---|---|---|---|---|
| Li, et al. 20096 | Individual cohort-study/2a | White-test vs. No test | 63 vs. 74 | Germany |
| Liu, et al. 20128 | Low quality RCT/2a | White-test vs. No test | 53 vs. 54 | China |
| Leelawat, et al. 20129 | Individual cohort-study/2a | White test + Saline test in the same group of patients | 30 | Thailand |
| Linke, et al. 2014 Original data in this manuscript. | Individual cohort-study/2a | White-test vs. No test | 32 vs. 93 | Germany |
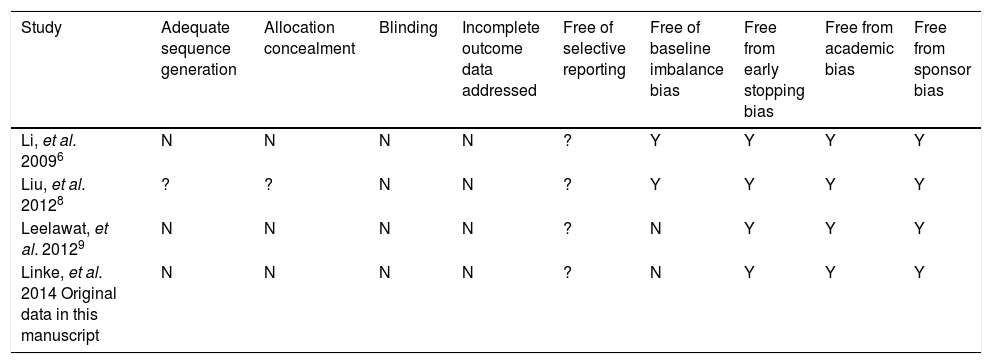
Quality assessment of studies included.
| Study | Adequate sequence generation | Allocation concealment | Blinding | Incomplete outcome data addressed | Free of selective reporting | Free of baseline imbalance bias | Free from early stopping bias | Free from academic bias | Free from sponsor bias |
|---|---|---|---|---|---|---|---|---|---|
| Li, et al. 20096 | N | N | N | N | ? | Y | Y | Y | Y |
| Liu, et al. 20128 | ? | ? | N | N | ? | Y | Y | Y | Y |
| Leelawat, et al. 20129 | N | N | N | N | ? | N | Y | Y | Y |
| Linke, et al. 2014 Original data in this manuscript | N | N | N | N | ? | N | Y | Y | Y |
N: no. Y: yes. ?: unknown.
An analysis of 125 consecutive cases of major hepatic resections in accordance with the Brisbane classification (> 3 segments) carried out at Frankfurt University Clinic between 2011 and 2012 was performed.15 Groups were divided into patients undergoing resection in which an intraoperative White-test was applied and in those that did not receive a White-test routinely during operation. Patient data included performance of White-test, intraoperative identification of biliary leakages and the postoperative incidence of biliary leakage in accordance with the definition of the International Study Group for Liver Surgery (ISGLS).16 Data were included in the meta-analysis.
Statistical analysis and data extractionData investigating the primary and secondary outcome measures of the selected publications were collected as total numbers from the original publications. The dichotomous outcomes were arranged in fourfold-tables for each independent study. Using the Mantel-Haenszel method assuming a fixed effects model an analysis of pooled data was performed. Odds Ratios of the meta-analysis were displayed with 95% confidence intervals (95% CI) in Forest plots. Analyses were performed with Review Manager (RevMan), version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
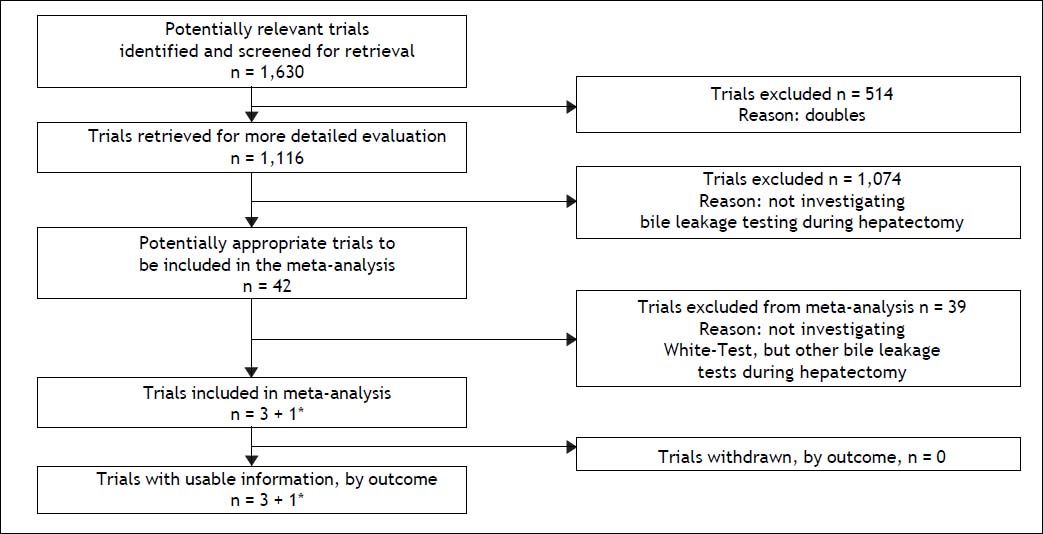
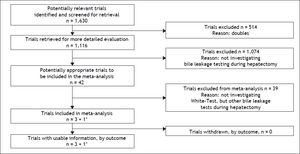
ResultsResults of the literature searchWith the preferred terms used we identified 1,630 articles. After removal of duplicates, 1,116 articles remained, of which 42 investigated the intraoperative use of biliary leakage testing. Removal of those not investigating the White-test (introduced in 2006 by the Essen-group of Silvio Nadalin6) and reviews led to a total of 3 studies plus our own data which were suitable for analysis of primary and secondary outcome measure. For the analysis of primary and secondary outcome 4 studies were included. Notably, 1 study was only included in the secondary outcome analysis, and our own data were included both analyses. The selection diagram of the PRISMA-statement is elaborated in figure 1.
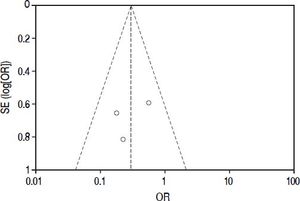
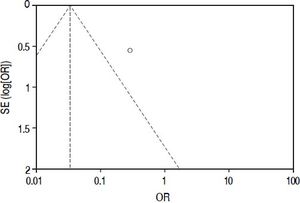
Included studiesFor the primary outcome measure “incidence of postoperative biliary leakage” 3 studies (including our original data) were chosen. Li, et al. originally described the method in 2009. They performed an unmatched cohort study of two consecutive cohorts of patients in which the incidence of biliary leakage was determined intra-operatively and after operation.6 Patients were not randomized. Liu, et al. performed a randomized controlled trial of Whitetest vs. No test describing a simple randomization procedure according to the admission sequence. Both, major and minor hepatectomies were included. Unfortunately, no adequate power calculation for sample size was provided.8 Finally, data from a cohort of consecutive 125 patients undergoing major hepatectomies at our center were included in the analysis. White-test was performed at the discretion of the surgeon and not systematically applied in the investigated area. We only included data in the White-test group, which could be undoubtedly identified from surgical protocols and reports. For secondary outcome measure, the 3 mentioned studies were included. Additionally, a study performed by Leelawat, et al. was chosen which showed, that by testing with saline intraoperatively first, followed by White-testing in the same patients, a larger number of patients with intraoperative biliary leakage could be identified.9 In total 369 patients (148 in the White-test and 221 in the No test group) were included in the primary outcome measure analysis. There was a statistically highly significant difference favoring White-Testing during hepatectomy to reduce the incidence of postoperative biliary leakage [OR: 0.30 (95% CI: 0.14; 0.63]; p = 0.002). The secondary outcome measure showed also a superiority of White-testing in comparison to no testing, respectively saline testing in one study [OR: 0.03 (95% CI: 0.02; 0.07]; p < 0.00001). Basic data of included studies are displayed in table 1. Forest-plots of primary and secondary outcome measures are displayed in figures 2-5.
Excluded were studies that did not use an intraoperative biliary leakage test and studies that tested other biliary leakage tests without using the White-test, like solely application of methylene-blue, indocyanine-green application, saline-application and others). Since the White-test is a recently introduced technique in hepatic surgery the number of analyzed studies was low including non-randomized cohort trials.
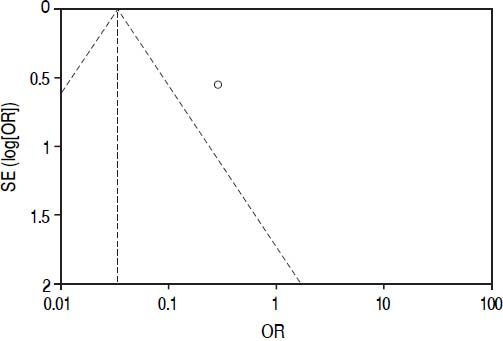
Quality assessment and risk of biasThe overall quality of existing evidence is medium to low. There was only one randomized controlled trial (RCT), however sample-size was not based on a statistical power-calculation. The other studies included were cohort-studies of acceptable size and a clear definition of treatment and control groups. Based on the definition of evidence levels provided by the Centre of Evidence in Oxford, all included studies including the low quality RCT must be classified as evidence level 2b: individual cohort-study (including low-quality RCT).14 Risk of bias was assessed according to the guidelines of the Cochrane Group (Cochrane Handbook Version 5.1.0, section 8). As there are no randomized controlled trials, one must assume a relatively high risk of bias throughout the studies. This accounts particularly for the selection of the treatment modality. There was no blinding for data assessment described, so detection bias can be at high risk. The lack of published study protocols in advance precludes exclusion of selective outcome reporting. Moreover, a quality assessment of study performance cofounders was performed and outlined in table 2. This assessment detects an overall low quality of the performed studies.
DiscussionSummary of main resultsThe White-Test for the identification of biliary leakages intraoperatively and the reduction of the incidence of postoperative biliary leakage showed a significant reduction in the incidence of postoperative biliary leakage [OR 0.30 (95% CI: 0.14, 0.63); p = 0.002]. The test is very good in detecting intraoperative biliary leakage due to its easy repeatability. It is superior to simple observation of the resection surface [OR 0.03 (95%CI: 0.02, 0.07); p < 0.00001].
Overall completeness and applicability of evidenceThe findings of this systematic review and meta-analysis of White-test application during hepatic surgery to detect biliary leakage and thus reduce the incidence of post-hepatectomy biliary leakage are relevant for a better outcome in hepatobiliary surgery. Biliary leakage, with reported rates of up to 30% after major hepatectomy, special indications or complicated segmentectomies and atypical resections is one of the major contributor to morbidity in hepatobiliary surgery.2,4,5 The test is easily to perform during surgery, cheap and repeatable at the surgeon’s choice. Therefore, cost-benefit estimation supports the use of the test during hepatic surgery.
Quality of evidenceThe overall quality of evidence is at a medium level as outlined in the results section, which has some implication on future research, which will be outlined in the conclusion section. However, quality of the included studies was overall low: first, there is only one trial that performed a low quality randomization procedure. Second, 3 out of 4 analyzed studies were cohort studies, which limits the power of the findings significantly.
Agreements and disagreements with other studies or reviewsCurrently, Wang, et al. published a review and meta-analysis of all available bile leakage tests.10 The findings were similar and pointing out that the White-test is the best choice for the test when performed. A problematic aspect which is in disagreement with our review is the classification of evidence following proposed scoring systems for classification of publications.11,12 The quality of some publications thus is overestimated and may have some suggestive effects on the uncritical reader. However, the White-Test seems to be the most promising tool to detect biliary leakage intraoperatively and avoid postoperative bile leakage in the conglomeration of low evidence. Therefore, our findings may be regarded as promising.
ConclusionsImplications for practiceCurrently, there is one RCT investigating Whitetesting during hepatic surgery delivering promising results, which are confirmed by the findings of cohort-studies. White-test should be performed during hepatic surgery to detect biliary leakages and reduce the incidence of post-hepatectomy biliary leakage.
Implications for researchFor the future a RCT with adequate sample-size calculations is necessary to create solid evidence for the White-test. We are currently working on a protocol for a randomized controlled trial in hepatic surgery of White-test vs. No test after hepatic surgery to reduce the incidence of biliary leakage to increase quality in hepatobiliary surgery. As outlined in the current health strategy of the German government, only the increase of high-quality medical performance, which includes removal of medical insecurities, will help to improve care for our patients.
Author’S Contributions- •
RL: performed literature search, collected data and wrote parts oft he manuscript.
- •
FU: generated idea and gave significant intellectual input.
- •
WOB: generated idea and gave significant intellectual input.
- •
AAS: generated idea, performed literature research and analysis and wrote parts of the manuscript.