Background. The effectiveness of nucleoside analogue (NA) treatment in patients with chronic hepatitis B (CHB) -associated liver failure is still controversial. Severe lactic acidosis has been reported during entecavir (ETV) treatment in patients with impaired liver function.
Aim. To investigate the rescuing efficacy and safety of ETV in patients with CHB-associated liver failure.
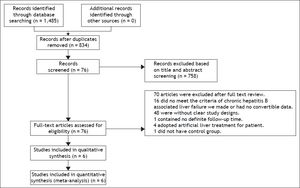
Material and methods. A literature search was carried out to collect articles dated up to December, 2013 on ETV therapy for patients with CHB-associated liver failure. Risk ratio (RR) and mean difference (MD) were used to measure the effects. Survival rate was used as the primary efficacy measure. The safety of ETV was assessed.
Results. Six randomized controlled trials were selected. The overall analysis revealed ETV significantly improved survival at 4 weeks (RR = 1.35; 95% CI [1.16, 1.57]; p < 0.0001), 8 weeks (RR = 1.33; 95% CI [1.07, 1.64]; p = 0.009), 12 weeks (RR = 1.68; 95% CI [1.24, 2.28]; p = 0.0008). Pooled data also showed beneficial effects of antiviral therapy compared with control for HBV DNA negative change (RR = 5.35; 95% CI [2.06, 13.88]; p = 0.0006), TBIL and PTA improvement (TBIL: MD = −69.36; 95% CI [−134.37, −4.36]; p = 0.04. PTA: MD = 16.26; 95% CI [8.59, 23.94]; p < 0.0001). No adverse effect was identified in the examined studies.
Conclusion. Our results showed that antiviral therapy with ETV improved the short-term survival of patients with CHB-associated liver failure. In addition, ETV was well tolerated during the treatment period. Further studies are still needed to strengthen these results.
Chronic hepatitis B (CHB), caused by the hepatitis B virus (HBV), is a serious health problem worldwide, especially in China, and other parts of Asia.1 Patients with chronic HBV infection are at an increased risk of developing liver cirrhosis and hepatocellular carcinoma.2 In some cases, patients may develop severe acute exacerbations, resulting in liver failure and death. Liver failure is the inability of the liver to perform its normal synthetic, metabolic, excretory and biotransformation functions, and usually manifests as coagulopathy, jaundice, ascites and hepatic encephalopathy.3 In China, HBV infection is the leading cause of liver failure, and HBV-induced liver failure is usually severe, with a high mortality.4 Current treatments for liver failure include supportive and symptomatic treatment-based comprehensive treatment, artificial liver support systematic treatment, and liver transplant.5 Liver transplantation is the most effective therapy for liver failure;6,7 however, it is limited by the availability of donor organs and high medical expense.
As we know, oral antiviral agents, nucleoside analogues (NAs), including lamivudine (LAM), adefovir dipivoxil (ADV), entecavir (ETV), telbivudine (LdT), and tenofovir disoproxil fumarate (TDF), have been licensed for the treatment of CHB patients.2,8 Recently NAs treatment has been proven effective in improving the status of patients with severe decompensated chronic liver disease and acute-on-chronic liver failure (ACLF) related to CHB.9–12 However, other studies found that NAs treatment did not improve the short-term prognosis of patients with HBV-associated liver failure.13,14 What’s even worse is that ETV treatment has been reported to be associated with increased short-term mortality in patients with severe acute exacerbation of CHB.15 In Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver,16 NAs were recommended as antiviral treatment. However, the level of evidence is only grade 3, meaning it is only based on the experience and comments made by specialists or authorities. Thus, the benefits of NAs therapy for this condition remain controversial.
Currently, the four types of nucleoside drugs available in the Chinese market include LAM, LdT, ETV, and ADV. ETV is a potent nucleoside inhibitor of HBV polymerase with a high antiviral activity and a high genetic barrier to resistance, and it has been regarded as one of the first line drugs for hepatitis B.2 In Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update, ETV is recommended as the agent of choice for patients with obvious or impending hepatic decompensation.17 However, severe lactic acidosis during treatment of CHB with ETV has been reported, occurring more often in patients with impaired liver function, especially in those with high model for end stage liver disease (MELD) scores and multi-organ failure.18 Thus, the safety of ETV remains a concern for clinical doctors treating patients with liver failure. In this paper, we selected randomized controlled trials (RCTs), and investigated the efficacy and safety of ETV in the treatment of patients with CHB associated liver failure by performing a systematic review and meta-analysis.
Material and MethodsInclusion and exclusion criteriaWe included studies from randomized controlled trials that compared ETV treatment with no antiviral treatment for patients with CHB-associated liver failure. According to the Prevention and treatment scheme for virus related hepatitis released in 2000,19 and Guidelines for the diagnosis and treatment of liver failure released in 2012,4 we made a criteria for the patients with CHB-associated liver failure in the selected studies.
Studies were included when the patients met the following criteria:
- •
Previous diagnosis of CHB or HBV-associated cirrhosis.
- •
Rapidly deepening jaundice, with total bilirubin (TBIL) 10 times greater than the upper limit of normality (≥ 171 μmol/L or 10 mg/dL), or a daily increase ≥ 17.1 μmol/L or 1 mg/dL.
- •
Hemorrhagic tendency with international normalized ratio (INR) ≥ 1.5 or prothrombin activity (PTA) ≤ 40%.
- •
- •
Interventional measure: routine comprehensive treatment in the control group; ETV (0.5 mg/d) combined with routine comprehensive treatment in the test group; and
- •
The neutrality and comparability of the two groups studied in terms of age, gender, and other biological or chemical predictors.
Exclusion criteria were:
- •
Patients with superinfection with hepatitis A, C, D, and E virus, Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, and others;
- •
Patients with other causes of chronic liver failure, like drug-induced liver injury, auto-immune liver disease, alcoholic liver disease, and inherited metabolic disease, et al;
- •
Patients with malignant tumors and severe blood anomalies;
- •
Patients receiving antiviral therapy at the time recruited to the study or having received antiviral therapy in 6 months before the studies;
- •
Patients receiving artificial liver treatment during the studies;
- •
Non-convertible or unusable data in literature; and
- •
Literatures not published as full text.
We searched Pubmed, MEDLINE, EMBASE, Cochrane Library, the Chinese BioMedical Literature (CBM), Chinese National Knowledge Infrastructure (CNKI), Chinese Technological Journal of Database (VIP) and Wanfang databases for eligible articles published up to December, 2013 without language and publication restrictions. To search for publications, we applied a free key word or mesh word search with the following terms: severe hepatitis B, chronic severe hepatitis B, liver failure, hepatic failure, acute on chronic liver failure, acute exacerbation, severe acute exacerbation, hepatitis B virus, hepatitis b virus infection, HBV, entecavir, ETV, baraclude, nucleoside analog* and nucleotide analog.* In addition, a manual search of abstracts of international liver meetings, reference lists of retrieved articles and qualitative topic reviews was also performed.
Indicators of therapeutic efficacyThe primary efficacy endpoint was survival rate. Secondary efficacy endpoints were HBV DNA negative change rate, TBIL and PTA changes. The safety of ETV treatment during the clinical course of liver failure was also assessed.
Data extraction and quality assessmentTwo reviewers (Xiaoguo Zhang and Lu Liu) independently selected the studies and extracted the data and outcomes according to the inclusion and exclusion criteria. In cases of disagreement between the two reviewers, a third reviewer (Shijun Chen) examined the data and discussed the choices with the two initial reviewers. The data were incorporated only when the three reviewers reached a consensus. The information collected included basic information on the studies, sample sizes, receiver characteristics (gender, age, TBIL, PTA, HBV DNA, etc.), and results.
Methodological quality assessment of the included studies was performed using the Cochrane risk of bias tool (Random sequence generation, Allocation concealment, Blinding of participants and personnel, Blinding of outcome assessment, Incomplete outcome data, Selective reporting, Other sources of bias) as described in the Cochrane Reviewer’s Handbook 5.1.0.
Statistical analysisAnalysis was performed with review manager version 5.2.7 (Rev Man 5.2.7 from Cochrane Collaboration). Risk ratio (RR) and 95% confidence interval (95% CI) were used to compare survival and HBV DNA negative change rates. Mean difference (MD) and 95% CI were used to compare TBIL and PTA changes. The heterogeneity of the data collected was measured using Cochran’s Q test (Chi-square χ2 test) and tests. The fixed or random effect model was used depending on the presence or absence of significant heterogeneity. In our meta-analysis, P > 0.10 in Cochran’s Q test and I2 < 25% were considered indicators of no or low-level heterogeneity, and the fixed effects model was adopted when the data across studies was pooled. Otherwise, the random effects model was used. For all tests except heterogeneity, P < 0.05 was considered statistically significant. Funnel charts, Egger’s test and Begg’s test were used to detect possible publication biases. Sensitivity analysis was performed to evaluate the validity and reliability of the primary meta-analysis.
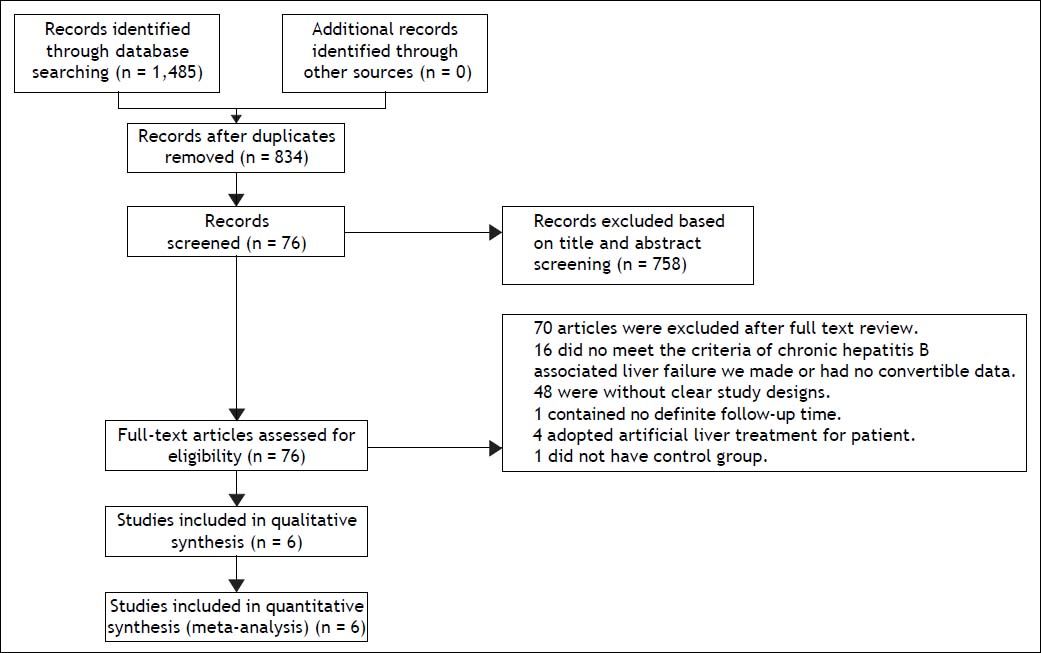
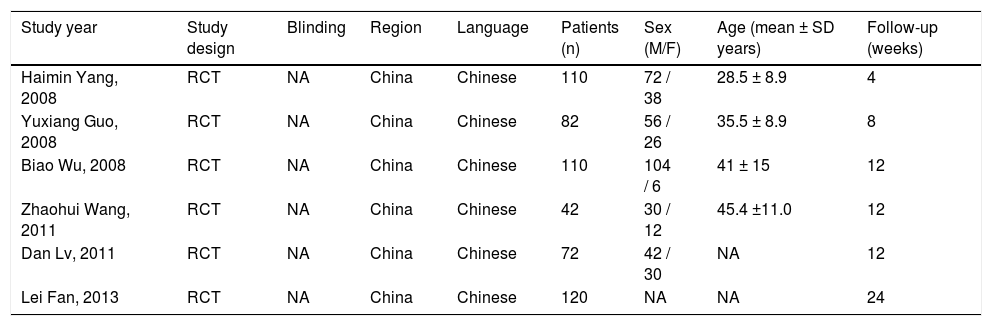
ResultsCharacteristics of the included studiesA total of six studies comprising 536 patients fulfilled the criteria for this systematic review and meta-analysis.21–26 The strategy is summarized in figure 1. All selected studies originated from mainland China and were published in Chinese. One26 study had three-arm design with two test groups: one using ETV, and the other using LAM. All patients were given routine comprehensive treatment, including: intensive care monitoring, nutritional supplementation, intravenous drop infusion of albumin and plasma, maintenance water, and electrolyte and acid-base equilibrium. Detailed characteristics of the included studies are shown in table 1.
Characteristics of the six studies included in the meta-analysis.
| Study year | Study design | Blinding | Region | Language | Patients (n) | Sex (M/F) | Age (mean ± SD years) | Follow-up (weeks) |
|---|---|---|---|---|---|---|---|---|
| Haimin Yang, 2008 | RCT | NA | China | Chinese | 110 | 72 / 38 | 28.5 ± 8.9 | 4 |
| Yuxiang Guo, 2008 | RCT | NA | China | Chinese | 82 | 56 / 26 | 35.5 ± 8.9 | 8 |
| Biao Wu, 2008 | RCT | NA | China | Chinese | 110 | 104 / 6 | 41 ± 15 | 12 |
| Zhaohui Wang, 2011 | RCT | NA | China | Chinese | 42 | 30 / 12 | 45.4 ±11.0 | 12 |
| Dan Lv, 2011 | RCT | NA | China | Chinese | 72 | 42 / 30 | NA | 12 |
| Lei Fan, 2013 | RCT | NA | China | Chinese | 120 | NA | NA | 24 |
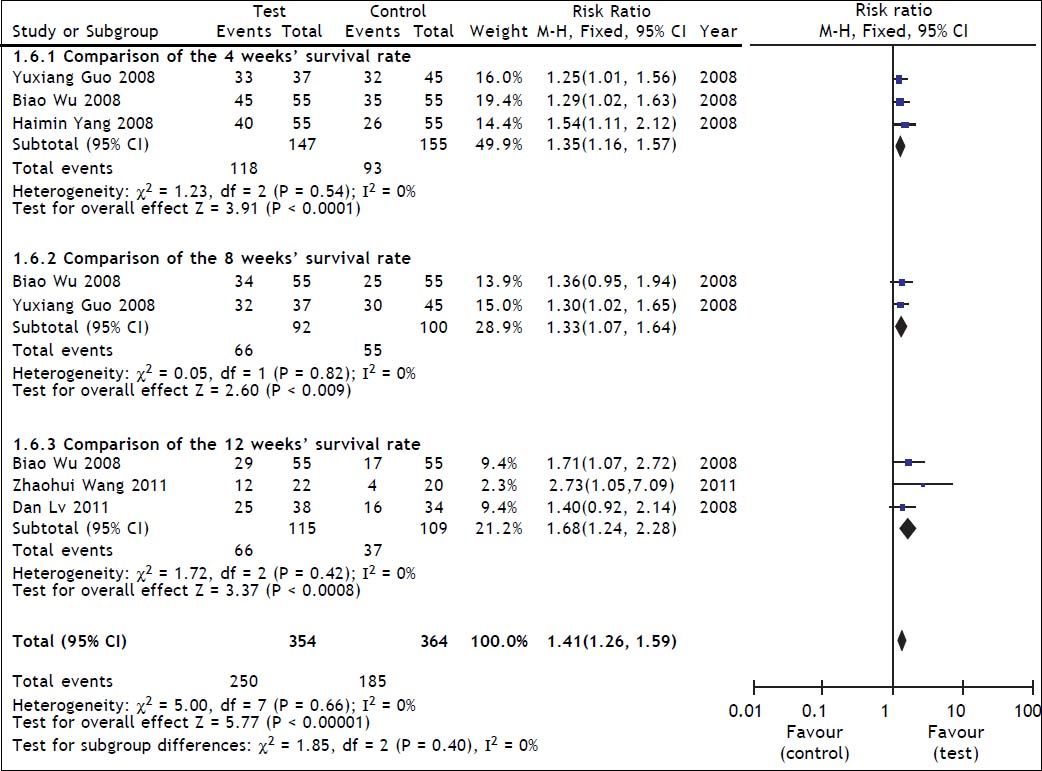
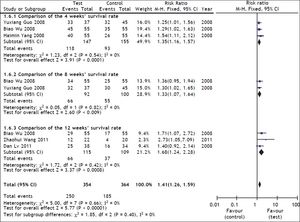
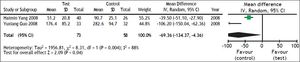
Survival rate was used as the primary efficacy endpoint in the selected studies with 267 cases in the test group and 269 cases in the control group. In the heterogeneity test, χ2 = 5.00, df = 7, P = 0.66, and I2 = 0% (Figure 2), suggesting that there was no significant variability among the included studies. Therefore, the fixed effect model was used to combine the effect measures using RR as indicator (RR = 1.41), and 95% CI was (1.26, 1.59). In the test for the overall effect, Z = 5.77 and P < 0.00001, suggesting that ETV treatment in patients with CHB-associated liver failure improved the survival rate.
A heterogeneity test in the subgroup analysis was conducted during the treatment course (4 weeks, 8 weeks and 12 weeks). Of the selected studies, three21–23 reported 4-weeks’ survival for 302 patients (147 patients received ETV). The assessment of heterogeneity produced P = 0.54 in Cochran’s Q test and I2 = 0%, which indicated that there was no significant variability between these studies. The fixed effect model was therefore used, and the estimated pooled RR for these studies showed a significant increase in survival rate for patients receiving antiviral therapy (RR = 1.35; 95% CI [1.16, 1.57]; p < 0.0001).
Two studies22,23 reported 8-weeks’ survival for 192 patients (92 patients received ETV). The assessment of heterogeneity produced p = 0.82 in Cochran’s Q test and I2 = 0%, which indicated that there was no significant variability between these 2 studies. The fixed effect model was therefore used, and the estimated pooled RR for these studies showed a significant increase in survival rate for patients receiving antiviral therapy (RR = 1.33; 95% CI [1.07, 1.64]; p = 0.009).
Three studies23–25 reported 12-weeks’ survival for 224 patients (115 patients received ETV). The assessment of heterogeneity produced P = 0.42 in Cochran’s Q test and I2 = 0%, which indicated that there was no significant variability between these studies. The fixed effect model was therefore used, and the estimated pooled RR for these studies showed a significant increase in survival rate for patients receiving antiviral therapy (RR = 1.68; 95% CI [1.24, 2.28]; p = 0.008).
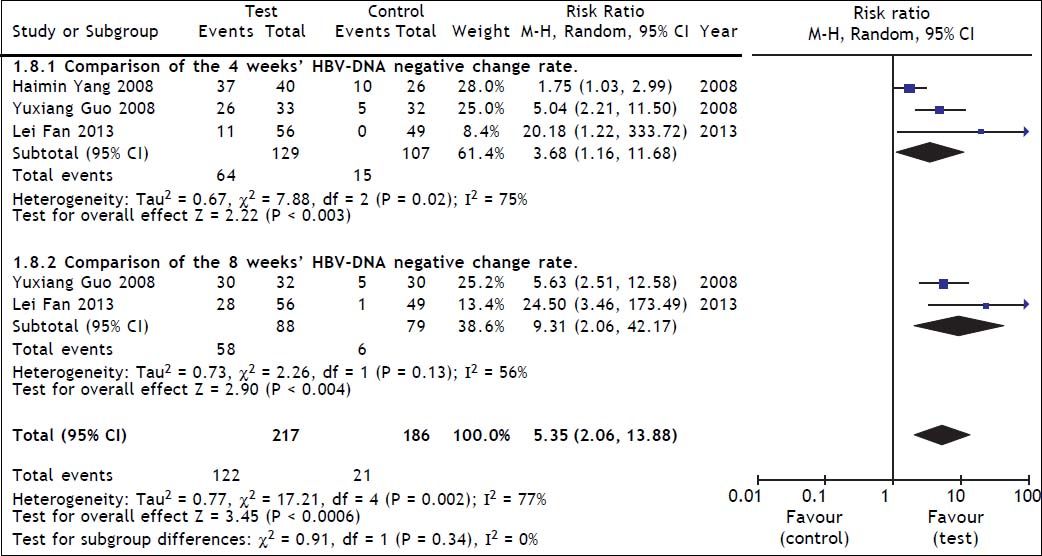
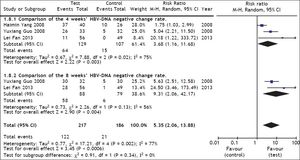
Comparison of HBV DNA negative change rate between test and control groupsThree studies21,22,26 reported HBV DNA negative change at 4 weeks, and two22,26 studies reported HBV DNA negative change at 8 weeks. A heterogeneity test showed that χ2 = 17.21, P = 0.002, and I2 = 77%, suggesting the existence of heterogeneity among these studies. The random effect model was therefore used to combine the overall effects, and the estimated pooled RR showed a high HBV DNA negative rate for patients receiving ETV therapy (RR = 5.35; 95% CI [2.06, 13.88]; p = 0.0006) (Figure 3). Heterogeneity in the subgroup analysis existed during the treatment course (4 and 8 weeks), so the random effect model was used, and the resulting RRs were 3.68 and 9.31 respectively, and the 95% CIs were (1.16, 11.60) and (2.04, 42.17) respectively. In the test for the overall effect, Z = 3.45 and P = 0.0006, which indicated a significant statistical difference. Thus, ETV treatment significantly inhibited HBV DNA replication.
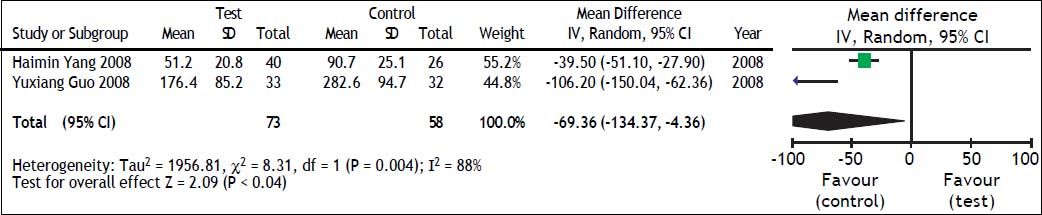
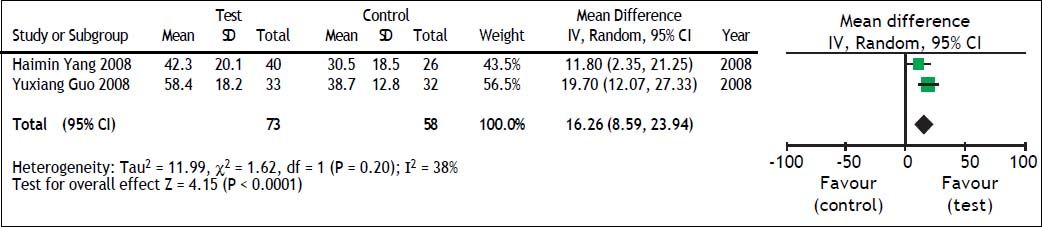
TBIL and PTA comparison between test and control groupsWe also evaluated the impact of antiviral therapy on TBIL and PTA changes. There were only two studies21,22 that reported TBIL and PTA changes at 4 weeks and heterogeneity was found among these groups (TBIL: χ2 = 8.31, df = 1, P = 0.004, and I2 = 88% [Figure 4]; PTA: χ2=1.62, df = 1, P = 0.20, and I2 = 38% [Figure 5]). The random effect model was therefore used to combine the overall effects using MD as the indicator. Our results showed that ETV decreased the TBIL level (MD = −69.36; 95% CI [−134.37, −4.36]; p = 0.04) and increased PTA levels (MD = 16.26; 95% CI [8.59, 23.94]; p < 0.0001) at the 4 weeks after antiviral therapy. Lei Fan’s study26 reported TBIL and PTA changes at 24 weeks. In that study, liver function in the test group was significantly better than that in the control group.
SafetyNo studies reported patients developing drug-related adverse events or drug related viral mutation.
Sensitivity analysisTo confirm the stability of the primary analysis, we conducted a sensitivity analysis by excluding studies one by one and found that the overall survival rate did not change significantly with the exclusion of any single study.
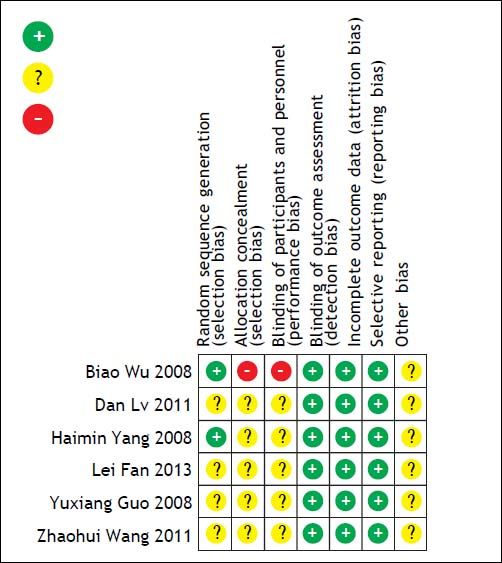
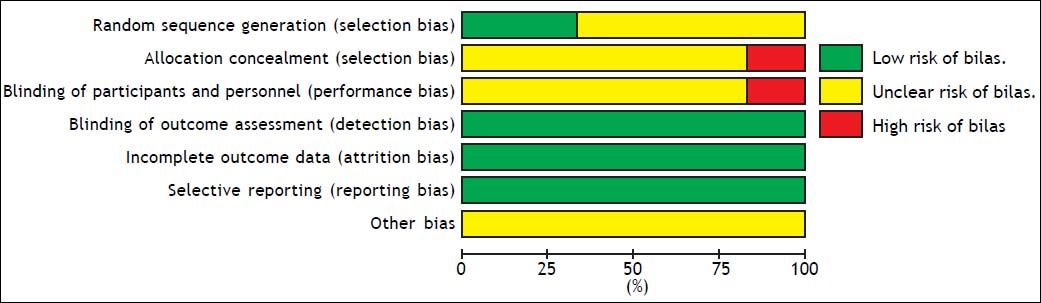
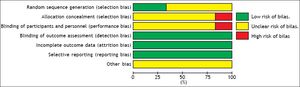
Risk of bias in included studiesThe overall quality of the studies included in this meta-analysis was suboptimal. Each of the six studies was a RCT, of which four did not report how the allocation sequences were generated. Five studies did not report the methods of the allocation concealment, and one study took an open random allocation schedule. Five studies did not report the blinding of the study participants and personnel. Because different follow-up times and different outcomes were reported, often without full statistical details, it was not possible to meta-analyze all the data. Risk of bias graph and summary are shown in figures 6 and 7.
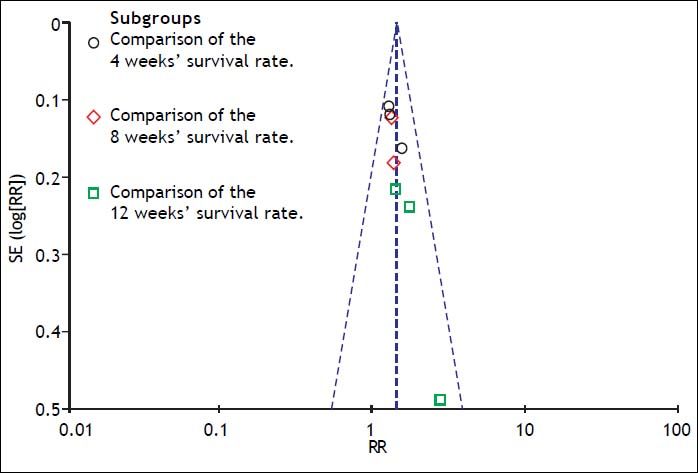
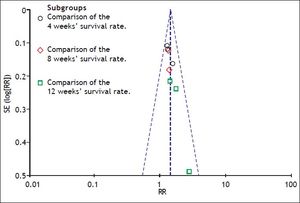
A funnel plot of the studies used in the meta-analysis reporting on the survival rate is shown in figure 8. Egger’s test and Begg’s test were also used to evaluate the possibility of publication bias. No evidence of publication bias was found in our study.
DiscussionCHB-associated liver failure, once named severe chronic hepatitis B, represents one of the most difficult to treat liver diseases with a high mortality rate. The mechanisms involved in this disease are rather complicated and remain unclear, but one of the important known mechanisms is an overactive immune response, particularly the cytotoxic T lymphocyte reaction to infected hepatocytes, due to the high level of HBV replication and protein antigen expression on target cell surfaces. This mechanism is necessary for HBV clearance and causes significant apoptosis and necrosis of hepatocytes.2,27–29 In Zhao’s study on the cause and outcome of chronic and acute liver failure, HBV replication and mutation were the primary causes of severe hepatitis B.30 Therefore, a reasonable solution for treating CHB-associated liver failure is to inhibit HBV replication within the body and relieve immune hyperactivity using antiviral drugs.
LAM has been reported to decrease HBVDNA levels in the serum, improve liver function and enhance survival rate of patients with severe CHB.31 There is no doubt that ETV is superior to LAM in the treatment of CHB,32 but is ETV also effective in treatment for severe CHB or CHB-associated liver failure? Several reports have reviewed the use of ETV in the treatment of HBV-related decompensated liver disease, and ETV is considered to be one of the first-line therapies for patients with this disease.33–35 A retrospective matched cohort study by Ke Ma, et al.36 evaluated the survival rates of patients with Hepatitis B related ACLF receiving ETV treatment and found that the 1 and 3 month survival rates of patients in the ETV treated group (n = 124) were 72.58 and 61.29%, respectively, significantly higher than that in the NA free group (n = 124), which were 53.23 and 45.97%, respectively. In Tianyan Chen’s report on the efficacy of NA treatment in patients with HBV-related ACLF,37 42 patients were treated with 0.5 mg ETV daily (ETV group), and 34 patients did not take any NA (non-NA group). After 3 months of treatment, the non NA group had a significantly higher mortality compared with the ETV group (64.7 vs. 33.3%, χ 2 = 7.163, P = 0.007). Bingliang Lin’s study38 showed that ETV treatment for patients with acute on chronic hepatitis B liver failure significantly improved disease severity scores with a marked reduction in mortality and suppression in HBV DNA to undetectable levels at week 48. Moreover, the survival benefit was noted in ETV treated patients as early as week 3.
However, other studies drew different conclusions. Jun Chen13 collected data on 129 chronic severe hepatitis B patients: 55 were treated with ETV, and the remaining 74 were not treated with NAs. The results showed that although ETV greatly reduced HBV replication (P < 0.001), the MELD score, liver function and short-term survival rate showed no significant differences between test and control group. Yaoli Cui’s data14 also indicated that NAs (including ETV) treatment did not improve the short-term prognosis of patients with HBV-associated ACLF, although it was efficacious and safe in the management of HBV DNA levels. A study from Hong Kong even found that ETV treatment was associated with increased short-term mortality in patients with severe acute exacerbation.15
The effectiveness of ETV for treating chronic CHB-associated liver failure has been demonstrated by many trials, such as those discussed above. However, most were not randomized controlled trials. We wished to obtain a more stringent, conclusive result. In the present study, RCTs conducted before December 2013 were collected. Our primary pooling results demonstrated that ETV treatment improved the short-term survival rate of patients with CHB-associated liver failure. The strategy of subgroup analysis was adopted in our statistical analysis. We found that the 4, 8 and 12 week survival rates of the test groups were higher than those of the control groups. Thus, ETV appeared to improve the survival rate of patients at weeks 4, 8 and 12 during the treatment course. Because different outcomes were reported, often without full statistical details, we presented the HBV DNA negative change rate at weeks 4 and 8, TBIL and PTA changes at week 4. Our results showed that ETV inhibited HBV replication and improved liver function.
ETV can inhibit the activity of HBV DNA polymerase. This inhibition decreases HBV replication, thereby reducing the viral load in the liver and blood, decreasing the target antigen expression on the surface of liver cells and reducing CTL attack on infected liver cells. Lu’s study39 demonstrated that ETV could regulate the function of dendritic cells in CHB patients, so the immune modulation may be another mechanism by which ETV treatment improves survival rate of patients with CHB- associated liver failure. Thus, it appears reasonable to use ETV for treating CHB-associated liver failure.
To investigate the optimal timing for therapeutic efficacy of ETV for HBV-related ACLF in hepatitis B e antigen negative patients, 109 patients were collected by Yan Y40 and divided into three groups according to MELD score: high (≥ 30); intermediate (22-30); and low (≤ 22). When ETV was added to comprehensive therapy, the mortality of patients with a high MELD score was 95.83%, while the mortality of patients with a low MELD score was 3.23%. Yan Y’s study suggested that ETV treatment for CHB-associated liver failure should be initiated as early as possible.
The safety of ETV in the included studies was also assessed. No studies reported the development of drug-related adverse events, drug-related viral mutation or the need for dose modification or early discontinuation.
In the end, we are obliged to mention the several limitations of our meta-analysis. First, the number of included studies was small, and some did not include all of the outcome parameters which we wanted to analyze at the different follow-up time points. As mentioned above, NAs therapy has been recommended as antiviral treatment for patients with HBV-associated liver failure. It seemed unethical to perform a controlled trial using no antiviral treatment, which limited the conduct of RCTs. Second, the quality of included studies was not optimal. Most of the studies did not report how the allocation sequences were generated and did not report the methods of the allocation concealment. Risk of bias may therefore exist in the included studies. Third, all of the included studies were conducted in China, thus the conclusion may not be generalizable to other populations. Finally, although the assessment of publication bias was not significant in our analysis, the possibility of publication bias may exist in any research because negative and small sample studies are less likely to be published. These limitations have all affected the reliability of research results. Therefore, future studies should assess high-quality, well-designed, multicenter RCTs with larger sample sizes.
ConclusionIn conclusion, our results showed that antiviral therapy with ETV improved the short-term survival rate of patients with CHB-associated liver failure. In addition, ETV was well tolerated during the treatment period. Further studies are still needed to increase the weight of these findings.
Authors’ ContributionsStudy conception and design: SC and YA. Data acquisition and analysis: XZ, LL, MZ and SG. Writing of the paper: XZ. Critical revision: YD and SC. All authors read and approved the final manuscript.
AcknowledgmentsThe authors would like to thank the members of Prof. Shijun Chen’s Laboratory for their helpful discussion and critical reading of the manuscript.
Conflict of Interest StatementThe authors declare that they have no conflict of interests.