Renal and bone impairment has been reported in chronic hepatitis B (CHB) patients receiving long-term tenofovir disoproxil fumarate (TDF) therapy. This study aimed to assess the incidence of renal and bone impairment in CHB patients with long-term TDF therapy and to identify the changes in bone mineral density (BMD) and renal function in these patients after switching to entecavir (ETV) or tenofovir alafenamide (TAF).
Materials and MethodsThis retrospective study collected clinical data from CHB patients who received TDF monotherapy over 96 weeks. The changes in BMD and renal function were analyzed after 96 weeks of switching antiviral regimens (ETV or TAF) or maintenance TDF.
ResultsAt baseline, 154 patients receiving TDF monotherapy over 96 weeks were enrolled, with a younger median age of 36.75 years, 35.1% (54/154) of patients experienced elevated urinary β2 microglobulin and 20.1% (31/154) of patients had reduced hip BMD (T<-1). At week 96, among the 123 patients with baseline normal BMD, patients who maintained TDF (n=85) had experienced a decrease in hip BMD, while patients who switched antiviral regimens (n=38) experienced an increase (-13.97% vs 2.34%, p<0.05). Among patients with a baseline reduced BMD (n=31), the alterations in BMD were similar in patients who maintained TDF (n=5) and those who switched antiviral regimens (n=26) (-15.81% vs 7.35%, p<0.05). Irrespective of baseline BMD status, renal function decreased significantly in patients who maintained TDF and improved in patients who switched antiviral regimens.
ConclusionsYounger CHB patients on long-term TDF therapy are at high risk for bone and renal impairment, with the risk being reduced when switched to ETV or TAF.
The World Health Organization estimated that 296 million people had chronic hepatitis B in 2019, and 1.5 million people are newly diagnosed with hepatitis B virus (HBV) infection each year despite safe and effective vaccines [1]. HBV infection significantly increases the risk of hepatocellular carcinoma (HCC), and nearly one million patients die yearly from HBV-related end-stage liver disease and its complications [2,3]. Clinical studies have demonstrated that nucleos(t)ide analogues (NAs) can improve liver histology and reduce the risk of hepatic events by efficiently suppressing HBV DNA replication [4–8]. Regrettably, NAs cannot eliminate covalent closed-loop DNA (cccDNA) from hepatocytes, and serum clearance of hepatitis B surface antigen (HBsAg) rarely occurs during NAs treatment. Therefore, lifelong treatment is normally required [9]. Although NAs are generally safe and relatively free of significant side effects, a small proportion of patients have experienced safety complications after long-term use of NAs, such as tenofovir disoproxil fumarate (TDF) related bone and renal impairment [10]. These patients are always ignored in clinical practice, especially younger patients.
TDF is highly effective, generally well-tolerated, and not subject to the development of resistance, so it is widely used to inhibit human immunodeficiency virus (HIV) and HBV replication. However, several studies have reported that patients with HIV infection on long-term TDF develop renal impairment, with proximal tubular dysfunction as the main clinical manifestation [11,12]. Besides, some patients experienced a reduction in BMD [13]. Therefore, guidelines recommend using alternative NAs instead of TDF in older CHB patients or those at risk for bone and renal impairment [5,14]. However, a critical clinical concern is whether bone and kidney safety can be ignored in younger CHB patients.
With the occurrence of safety events during long-term use of TDF, we need more information about the side effects of first-line antivirals to select or switch to rational antiviral regimens for CHB patients at risk of bone and renal injury, especially in younger patients. This study aimed to investigate the incidence and age distribution of bone and renal injury in a cohort of CHB patients receiving TDF monotherapy for more than 96 weeks. Moreover, analyzing changes in renal function and BMD in this cohort after 96 weeks of maintaining TDF monotherapy or switching antiviral regimen to entecavir (ETV) or tenofovir alafenamide (TAF).
2Materials and Methods2.1Study designThis is a single-center retrospective study. All CHB Patients who attended West China Hospital from December 2018 to May 2019 and received TDF monotherapy for more than 96 weeks were recruited. Study subjects were followed for clinical outcomes for at least 96 weeks. After completion of initial BMD and renal function testing between December 2018 and May 2019, some patients voluntarily switched antiviral regimens (ETV or TAF), and some patients maintained TDF.
The process of patients switching antiviral regimens was detailed as follows. After completion of initial BMD and renal function tests, for patients with abnormal renal function or reduced BMD, some patients switched their antiviral regimen under the doctor's guidance, while others voluntarily maintained their TDF antiviral regimen due to cost and other issues. In addition, a proportion of patients with normal BMD and renal function voluntarily switched their antiviral regimen after consulting doctors about the advantages and disadvantages of different drugs. We analyzed the changes in BMD and renal function after 96 weeks of switching to an antiviral regimen (ETV or TAF) or maintaining TDF.
2.2PatientsThe inclusion criteria were as follows: (1) female or male ≥18 and ≤ 65 years of age, (2) receiving TDF monotherapy for more than 96 weeks at the time of presentation (December 2018 to May 2019). (3) completed initial BMD and renal function testing during the presentation (December 2018 to May 2019). (4) After initial BMD and renal function testing, patients were followed up at least every 12 weeks for a total follow-up time of more than 96 weeks and completion of a second BMD test (with intervals of at least 96 weeks).
The exclusion criteria for CHB patients were as follows: (1) receiving NAs combination therapy or other NAs before recruitment, (2) co-infected with hepatitis C virus and other hepatitis viruses or HIV, (3) chronic kidney disease and bone metabolism disease, (4) patients taking drugs that affect bone metabolism and renal function,(5) co-existed with chronic liver diseases, such as alcoholic liver disease and autoimmune liver disease, (6) being pregnant or having uncontrollable malignancies, (7) lost to follow-up and missing essential data, (8) less than 96 weeks of follow-up after switching to TAF or ETV.
2.3Outcome assessment and measurementThe time of the patient's initially completed BMD and renal function tests were set as the baseline for this study. Bone impairment was assessed by BMD (g/cm2) and T values, with T ≥ -1 as normal BMD and T < -1 as reduced BMD, with -1 > T > -2.5 as osteopenia and T ≤ -2.5 as osteoporosis. Renal impairment was assessed by glomerular function and tubular function. The glomerular function was assessed by creatinine and estimated glomerular filtration rate (eGFR), and the eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, the normal range of eGFR is ≥90ml/min/1.73m2. The tubular function was assessed by urinary β2-microglobulin and blood phosphorus. Serum alanine aminotransferase (ALT) normalization rate (male <50U/L, female <40U/L) and virological response rate were used to assess antiviral efficacy.
Serum biochemical indexes were measured according to standard procedures (Olympus AU5400, Olympus Corporation, Tokyo, Japan). Serum HBV DNA concentration was quantitatively determined using a Cobas TaqMan assay kit (Roche Diagnostics, Branchburg, NJ), with a lower limit of detection of 100 IU/ml. The DXA (Dual Energy X-ray Bone Densitometry) method was used to test the patients' bone density, and the hip joint was tested in this study.
2.4Statistical AnalysisContinuous numerical variables are expressed as the mean ± SD or median (interquartile range), and categorical variables are expressed as ratios. The χ2 test or Fisher's exact test was used for categorical variables to compare differences. For continuous variables, t-tests, one-way ANOVA, or non-parametric tests are used as appropriate. Logistic regression is used to identify independent risk factors. A P-value less than 0.05 is considered to indicate statistical significance. Statistical analyses are performed using IBM SPSS version 26.0 and GraphPad Prism 8.0 software.
2.5Ethical statementThe study was conducted in accordance with the Declaration of Helsinki of 1975. The study protocol was approved by the Ethics Committee of West China Hospital of Sichuan University and registered with the Chinese Clinical Trials Registry (ChiCTR2100049655), and informed consent was obtained from each patient.
3Results3.1Patient characteristicsBetween December 2018 and May 2019, a total of 588 CHB patients received TDF monotherapy for more than 96 weeks in West China Hospital, and 378 patients did not complete initial BMD testing, 17 patients had comorbidities such as hepatitis C and renal dysfunction, 3 patients were taking drugs that affect bone metabolism, 20 patients had previously received other NAs, 4 patients were lost to follow-up, 5 patients were followed up less than 96 weeks after switching antiviral regimens, and 7 patients did not complete the second BMD test at an interval of 96 weeks. The process of patient selection is shown in Fig. 1.
After the screening, 154 eligible patients were enrolled in this study, and they were relatively young, with a median age of 36.75 (30.65,45.65) years. Of these patients, 57.8% (89/154) were under the age of 40 years, 55.8% (86/154) were male, 3.2% (5/154) had hypertension, and 3.9% (6/154) had diabetes mellitus. At baseline, the duration of TDF monotherapy was 116 (108,144) weeks, 8.4% (13/154) of patients with eGFR below 90 ml/min/1.73m2, and 35.1% (54/154) of patients experienced elevated urinary β2 microglobulin. Total hip BMD at baseline was 0.939 ± 0.13 g/cm2, with 20.1% (31/154) of patients having decreased BMD(T<-1) and no patients experiencing osteoporosis (Table 1).
Patients characteristics.
Notes: Data are presented as n (%), median (first-third quartile) or mean ± SD ALT, alanine aminotransferase; ULN, lower limit of normal; eGFR, estimated glomerular filtration rate; BMD, bone mineral density; LLN, lower limit of normal.
Among patients with reduced BMD at baseline, 64.5% (20/31) were younger than 40 (Fig. 2a). Compared with patients with normal baseline BMD, patients with reduced BMD had lower serum phosphorus levels and a lower ratio of normal urinary β2-microglobulin (Fig. 2b/2c).
Patient characteristics at baseline. (a), Age distribution of patients with baseline reduced BMD and elevated urinary β2-microglobulin; (b), Comparison of serum phosphorus between patients with baseline reduced BMD and normal BMD; (c), Comparison of urinary β2-microglobulin between patients with baseline reduced BMD and normal BMD; (d), Comparison of serum phosphorus between patients with baseline elevated urinary β2-microglobulin and normal urinary β2-microglobulin; (e), Comparison of BMD between patients with baseline elevated urinary β2-microglobulin and normal urinary β2-microglobulin; (f), Analysis of independent risk factors for reduced BMD; Abbreviations: BMD, bone mineral density; ALT, alanine aminotransferase; ULN, lower limit of normal; TDF, Tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; ETV, entecavir; * P<0.05; ** P<0.01, *** P<0.001. **** P<0.0001.
Among the patients with elevated urinary β2-microglobulin at baseline, 59.2% (32/54) were younger than 40 years (Fig. 2a) and had lower serum phosphorus levels and lower hip BMD compared with patients with normal urinary β2-microglobulin (Fig. 2d/2e). Further multivariate analysis found that urinary β2-microglobulin positivity was an independent risk factor for reduced BMD (Fig. 2f).
3.2Bone and renal safety at week 96 in patients with baseline normal BMDAfter completion of initial BMD and renal function testing, of the 123 patients with normal BMD at baseline, 38 switched antiviral regimens, and 85 maintained TDF (Table 2). Patients who switched antiviral regimens were older than those who maintained TDF(P<0.05), and there was no difference between the two groups in sex or duration of dosing (P>0.05). At week 96, hip BMD decreased from 0.99(0.92,1.06) g/cm2 to 0.86(0.78,0.92) g/cm2 in patients maintained on TDF, with a mean change of -13.97% ± 9.09%, 70. 6% (60/85) of patients experienced a reduction in hip BMD of more than 10% (Fig. 3a, b), 49.4%(42/85) of patients progressed to osteopenia (T<-1), and none developed osteoporosis (T≤2.5) (Table 2). Among patients who switched antiviral regimens, hip BMD increased from 0.92(0.87,0.97) g/cm2 to 0.94(0.90,1.01)g/cm2 at week 96, with a mean change of 2.34% ± 7.20%, 63.2% (24/38) of patients experiencing an increase in BMD(Fig. 3a, b), which was significantly different from patients who maintained TDF (P<0.05) (Table 2).
Changes in BMD and renal function at week 96.
Notes: Data are presented as n (%), median (first-third quartile) or mean ± SD TDF, Tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; ETV, entecavir; ULN, lower limit of normal; eGFR, estimated glomerular filtration rate; BMD, bone mineral density.
Changes in BMD and renal function after week 96 of switching antiviral regimens. (a), Changes in BMD at week 96 in patients with baseline normal BMD; (b) Comparison of the degree of changes in BMD and eGFR in patients with baseline normal BMD after 96 weeks of switching antiviral regimens or maintaining TDF; (c), Changes in BMD at week 96 in patients with baseline reduced BMD; (d), Comparison of the degree of changes in BMD and eGFR in patients with baseline reduced BMD after 96 weeks of switching antiviral regimens or maintaining TDF; (e), Comparison of antiviral efficacy of ETV, TDF, and TAF; (f) Correlation analysis of changes in serum phosphorus and BMD. Abbreviations: BMD, bone mineral density; ULN, lower limit of normal; ALT, alanine aminotransferase; TDF, Tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; ETV, entecavir; * P<0.05; ** P<0.01, *** P<0.001. **** P<0.0001; ns, P>0.05.
Regarding renal function, there were significant differences between patients who maintained TDF and those who switched antiviral regimens (Table 2). At week 96, serum phosphorus increased in patients who switched antiviral regimens, with a mean change of 13.47%±23.34%, whereas serum phosphorus decreased in patients who maintained TDF with a mean change of -7.15% ± 16.03%. The percentage of abnormal urinary β2 microglobulin decreased from 55.2% (21/38) to 5.3% (2/38) in patients who switched the antiviral regimens, while it increased from 8.2% (7/85) to 32.9% (28/85) in patients who maintained TDF. Concerning the patient's eGFR, there was a slight decrease in patients who switched antiviral regimens (-1.78 ml/min/1.73m2 IQR (-5.82,5.07)), while a significant decrease occurred in patients maintaining TDF (-18.01 ml/min/1.73m2 IQR (-26.12, -9.11)), with 68.2% (58/85) experiencing a reduction more than 10% and 12.9% (11/85) more than 30% (Fig. 3b).
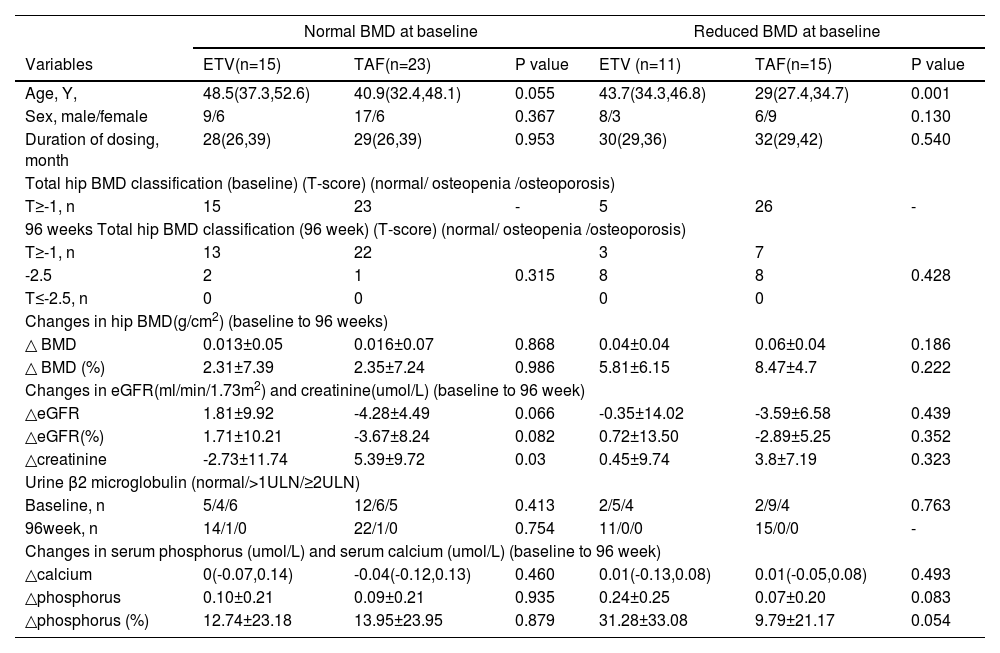
3.3Subgroup analysis in baseline normal BMD patientsAmong the 38 patients who switched to the antiviral regimen, 15 switched to ETV and 23 to TAF. There were no significant differences between the two groups in terms of age, sex, and duration of medication (P>0.05) (Table 3). At week 96, serum phosphorus and hip BMD increased in both groups, and the number of patients with abnormal urinary β2 microglobulin decreased (P>0.05). There was a slight decrease in eGFR in patients who switched to TAF (-4.28±4.49 ml/min/1.73m2) and an increase in eGFR in patients who switched to ETV (1.81±9.92 ml/min/1.73m2), but no statistical difference between the two groups (P>0.05).
Comparison of differences between patients who switched to ETV and TAF.
Notes: Data are presented as n (%), median (first-third quartile) or mean± SD TDF, Tenofovir disoproxil fumarate; TAF, tenofovir alafenamide; ETV, entecavir; ULN, lower limit of normal; eGFR, estimated glomerular filtration rate; BMD, bone mineral density
After completion of initial BMD and renal function testing, of the 31 patients with reduced BMD at baseline, 26 switched antiviral regimens, and 5 maintained TDF(Table 2). At week 96, we compared the alterations in BMD, the hip BMD increased from 0.80(0.76, 0.82) g/cm2 to 0.84(0.81, 0.87) g/cm2 in patients who switched regimens, with a mean change of 7.35%±5.41%, and 90.9% (25/26) of these patients experiencing an increase and 3.8% (1/26) experiencing a reduction, but the reduction range less than 3% (Fig. 3c, d). Hip BMD decreased from 0.82 (0.78, 0.84) g/cm2 to 0.70 (0.59, 0.77) g/cm2 in patients maintained on TDF, with a mean change of -15.81% ± 11.59%, which was significantly different from patients who switched antiviral regimens (Fig. 3c, d) (P<0.05).
Regarding the renal function (Table 2), similar to patients with normal baseline BMD, serum phosphate increased in patients who switched antiviral regimens and decreased in patients who maintained TDF (18.88%±28.39% vs. -14.39%±11.5% p<0.05). The proportion of patients with abnormal urinary β2-microglobulin decreased from 84.6% (22/26) to 0% (0/26) in patients who switched antiviral regimens. However, the proportion decreased from 80% (4/5) to 60% (3/5) in patients who maintained TDF, and the degree of urinary β2-microglobulin elevation was significantly higher than baseline. About eGFR, it increased in 84.6% (22/26) of patients who switched antiviral regimens, with a median change of -2.72 ml/min/1.73m2 IQR (-7.79, 3.13)). At the same time, it decreased significantly in patients who maintained TDF (-25.36 ml/min/1.73m2 IQR (-28.19 -7.5)) (Fig. 3d).
3.5Subgroup analysis in baseline reduced BMD patientsAmong patients with reduced BMD at baseline, 11 were switched to ETV, and 15 were switched to TAF (Table 3). We compared the differences between ETV patients and TAF patients. Patients who switched to TAF were younger than those who switched to ETV(P<0.05). At week 96, patients in both groups had significantly increased BMD and serum phosphorus, and the proportion of patients with abnormal urinary β2 microglobulin was significantly reduced. The two groups had no significant difference (P>0.05).
3.6Antiviral and biochemical response at week 96At baseline, 14.3% (22/154) of patients had ALT above the upper limit of normal (ULN), and 14.9% (23/154) were with positive HBV-DNA (Table 1). After 96 weeks of switching antiviral regimens, the TAF, ETV, and TDF groups maintained high biochemical and virological response rates, none experienced HBV breakthrough during the follow-up period, and there was no significant difference in antiviral efficacy between the three groups (P>0.05) (Fig. 3e).
3.7Correlation between the serum phosphorus and BMDOur study found that a decrease in serum phosphorus accompanied a decrease in BMD. Further correlation analysis showed that changes in serum phosphorus were positively correlated with changes in hip BMD (r2=0.535, p<0.05) (Fig. 3f).
4DiscussionGuidelines, including EASL and AASLD, have recommended TAF or ETV rather than TDF in older patients or at risk for bone and renal impairment. However, insufficient attention has been paid to bone and renal safety in younger CHB patients in clinical practice. The patients enrolled in our study were younger. The findings demonstrated a high incidence of bone and renal injury associated with TDF in younger CHB patients, with 64.5% (20/31) of patients with reduced BMD younger than 40 (Table 1). Thus bone and renal safety in younger CHB patients need significant attention.
Renal impairment due to TDF was initially reported in HIV patients. Under physiological conditions, proximal tubular secretion is the main pathway for the excretion of TFV from the body, with the organic anion transporter (OAT) and multidrug resistance-associated protein (MRP) transporter playing a key role [15]. Regarding the mechanism of renal injury caused by TDF, experiments have demonstrated that it is related to the massive accumulation of TFV in the renal tubules, leading to acute damage to the proximal tubular epithelium and mitochondria [16]. Urinary β2 microglobulin is a sensitive indicator of proximal tubular function, blood phosphorus is reabsorbed through the proximal tubule, and variations in serum phosphorus may reflect alterations in renal tubular function [17]. In our study, after receiving long-term TDF monotherapy, 35.1% (54/154) of CHB patients had developed elevated urinary β2 microglobulin at a younger age. Renal function and blood phosphorus further decreased in these patients after 96 weeks of continued TDF treatment. Therefore, it is definite that patients with CHB on long-term TDF therapy will develop renal dysfunction and cannot be ignored in younger patients.
Bone impairment is another problem in patients on long-term TDF therapy and is currently thought to be related to the nephrotoxicity of TDF, with hypophosphatasia as a potential mechanism. Clinical studies have demonstrated that reduced BMD in TDF -treated HIV patients is associated with reduced serum phosphate [13,18–21]. In our study, after receiving TDF monotherapy for more than 96 weeks, 20.1% of patients experienced a reduction in BMD at baseline. Further decreases in hip BMD was seen in patients who continued maintenance TDF treatment. In addition, there was a positive correlation between changes in serum phosphate and BMD, so it is suggested that the reduced BMD due to TDF is associated with hypophosphatasia that results from proximal renal tubular dysfunction. The previous study has demonstrated that HBV-infected patients are at increased risk of bone loss and osteoporosis [22]. In our study, 57.8% of patients were younger than 40 years. However, a higher percentage of hip BMD reduction occurred after long-term TDF treatment, suggesting that the risk of bone injury caused by TDF should not be underestimated, especially in younger patients.
Compared with TDF, TAF reduces the risk of side effects through liver targeting and higher plasma stability [23]. Clinical trials have demonstrated that in patients with CHB, TAF can exert similar antiviral efficacy and is significantly superior to TDF in bone and renal safety [24–28]. In our study, when patients switched antiviral regimens from TDF to TAF, the hip BMD, serum phosphate, and proportion of patients with normal urinary β2-microglobulin were increased. Therefore, consistent with most clinical studies, TAF was confirmed to be superior to TDF in bone and renal safety.
Our study also found that ETV had a higher bone and kidney safety profile than TDF. There are few studies on the bone and renal safety of ETV [28]. A multicenter prospective study in Italy reported increased eGFR and serum phosphate and a reduced risk of bone impairment in previously TDF -treated patients after switching their antiviral regimen to ETV therapy [29]. In our study, patients with CHB experienced increased BMD and renal function after 96 weeks of switching their antiviral regimen from TDF to ETV. Proximal tubular secretion is also the main route of ETV excretion from the body. However, ETV-related renal impairment is less commonly reported, which may be associated with multiple transport receptors involved in the excretion process [30].
In our study, we performed a subgroup analysis of ETV and TAF and found no significant difference between the two groups regarding bone and renal safety. Although both ETV and TAF showed good antiviral effects in this study, it is worth noting that although most clinical studies in the real world showed a complete virological response within a short period of ETV treatment, approximately 20% of patients on long-term ETV treatment develop low-level viremia (LLV) [31]. Given that the lower limit of detection for HBV-DNA in this study was 100 IU/ml, the possibility of LLV in patients receiving ETV cannot be excluded. Another clinical issue worth noting is that despite its good antiviral efficacy and bone and kidney safety, TAF is relatively expensive, and some patients cannot afford it. Therefore, we need to consider patients' financial ability when selecting antiviral regimens for them. For patients at risk of bone and renal injury and with limited financial resources, we can use ETV but need to be cautious about developing LLV. We can still select TDF treatment for patients without the risk of bone and renal injury.
There are some limitations to our study. Firstly, the sample size was relatively small. Second, it was a single-center retrospective study with bias in sample selection, and a multicenter prospective study is needed to confirm the results. Thirdly, there were too few indicators to assess bone and renal impairment, and we need more precise indicators.
5ConclusionsIn conclusion, CHB patients on long-term TDF are at high risk of bone and renal injury, especially in younger patients, and ETV or TAF is an option for at-risk patients. Before choosing a first-line antiviral drug in clinical practice, we need to assess the risk of bone and renal injury and select a reasonable antiviral regimen.
Data Availability StatementThe data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statementFa- Da Wang: Data curation, Writing – original draft. Jing Zhou: Data curation, Writing – original draft. Lan-Qing Li: Data curation, Writing – original draft. Yu-Jing Li: Data curation, Writing – original draft. Meng-Lan Wang: Data curation, Writing – original draft. Ya-Chao Tao: Data curation, Writing – original draft. Dong-Mei Zhang: Data curation, Writing – original draft. Yong-Hong Wang: Data curation, Writing – original draft. En-Qiang Chen: Conceptualization, Data curation, Writing – original draft.