Hepatitis E virus (HEV) is not routinely screened in blood banks in low- and middle-income countries, and no specific biomarkers of exposure to this virus have yet been identified. We aimed to identify HEV seropositivity and detect virus RNA among blood donors from Mexico to further correlate risk factors related to infection and levels of interleukin-18 (IL-18) and interferon-gamma (IFN-γ) as potential biomarkers.
Materials and MethodsThis cross-sectional, single-center study included 691 serum samples of blood donors obtained in 2019. Anti-HEV IgG and IgM antibodies were detected in sera and the viral genome was screened in pooled samples. A statistical comparison of risk factors for infection, demographic and clinical features was performed; IL-18 and IFN- γ values were tested in sera.
ResultsOf all the individuals, 9.4% were positive for anti-HEV antibodies and viral RNA detection was confirmed in one of the pools positive for anti-HEV. From the analysis of risk factors, age and having pets were statistically significant for anti-HEV antibody detection. Seropositive samples showed significantly higher IL-18 concentrations relative to samples from seronegative donors. Interestingly, IL-18 values were similar when HEV seropositive samples were compared to samples from clinically acute previously confirmed HEV patients.
ConclusionsOur findings highlight the need to follow up on HEV in blood banks in Mexico and underscore that IL-18 could represent a biomarker of HEV exposure.
Although the risk of transfusion transmitted Hepatitis E virus (HEV) infections by blood products has been described since 2004, HEV screening has become a popular topic in transfusion medicine in the last decade given the finding that infection, which was initially considered exclusively acute and self-limited, may progress to chronicity [1,2]. Despite the relevance of infection in middle- and low-income countries, including those in Latin America, most of the available literature regarding blood safety is still limited in these regions [2–4].
It is estimated that 20 million HEV infections occur each year, which is recognized as the main cause of acute viral hepatitis worldwide [2,3]. HEV is an RNA virus excreted in the feces of infected persons/animals; therefore, it is spread largely through contaminated water in regions with poor sanitary conditions. The virus is also transmitted by zoonosis and by the maternal-fetal route. Transmission of HEV through blood and derivatives is an emerging health risk. The seroprevalence of HEV in blood donors from developed regions ranges from five to thirty percent, and less commonly, viremic donors have been identified; HEV RNA is found in one of six hundred donors in the Netherlands, one in eight hundred in Germany and one in nine thousand in the United States [5].
Based on nucleotide sequences, HEV has been classified into eight genotypes, of which five infect humans. Infection with genotypes 1 and 2 results in acute infection, whereas infection with genotypes 3, 4 and 7 can progress to chronic disease mainly related to immunosuppression. For most people, HEV infection and disease is self-limited. However, pregnant women with hepatitis E may present with progression to liver failure. People with preexisting liver disease may undergo further hepatic decompensation with HEV infection [3,6].
When analyzing those countries in Latin America with an exponential growth of liver diseases, Mexico stands out as the highest [7,8]. HEV genotype 2 was first identified in an outbreak in Mexico in the late 1980s [9]. More recent investigations in the country have identified HEV genotype 3 infection in animals [10], and we reported HEV genotypes 1 [11] and 3 [12] in pediatric patients with acute hepatitis and in patients with chronic liver damage, respectively. Moreover, we previously reported a high seroprevalence of HEV in patients with liver disease in Mexico [13], along with an imbalance occurring in the cytokine inflammatory profile during the onset of acute infection [14]. Altogether, strongly suggests that hepatitis E is underestimated in Mexico and, supports the notion that the pathogenesis of HEV is complex and mostly relies on host immunity.
Multiple pro-inflammatory markers have been studied to characterize the onset of liver disease under distinct etiologies; of those markers, Interleukin-18 (IL-18) has been shown to be related to the Child–Pugh severity of liver injury in cirrhotic patients [15]. Therefore, this cytokine may be useful as a marker for liver disease. In fact, IL-18 has been identified in previous studies for chronic complications in the setting of HCV infections [16], where a significant upregulation of IL-18 expression in chronic infection with persistently normal Alanine amino transferase (ALT) levels has been reported [17]. IL-18 is an Interferon-γ (IFN-γ) inducing factor and, considering that one of the most important consequences of IFN-γ secretion is the activation of anti-viral responses, the finding of IL-18 and IFN-γ in the setting of viral diseases may be useful to characterize inflammatory statuses resulting from previous or current virus infections. In that sense, during HEV infection, animal models have demonstrated significantly higher levels of IL-18 and IFN-γ in virus-infected tissues [18]. Moreover, a study in HEV-infected patients indicated that liver disease outcome relies on an IL-18-dependent mechanism [19]. To date, no data regarding IL-18 and IFN-γ levels as potential hepatitis E biomarkers have been reported.
In Latin America, Mexico's situation is unique, with the circulation of three different HEV strains. However, HEV is not routinely screened in blood banks, and biochemical markers for liver disease are not tested in blood banks in the country. Therefore, our main objective was to identify hepatitis E seropositivity in blood donors to further correlate those risk factors present in participants and to identify HEV RNA in samples as well as the levels of IL-18 and IFN-γ as potential biomarkers of previous or current disease.
2Material and Methods2.1Study design, study population and sample collectionThis cross-sectional study included a total of 757 serum samples of candidates to donate blood obtained from the Blood Bank of the Hospital General Regional 45, Instituto Mexicano del Seguro Social (IMSS) Guadalajara, Jalisco, located in West Mexico, from August 5th to November 29th, 2019. All individuals were compliant with the blood donation protocol according to the official health legislation in Mexico.
2.2Demographic features and data collectionDemographic and clinical information, such as sex, age, weight, and size, and data regarding the identification of pathogens in blood from donors during the donation protocol were obtained from donor records in the blood bank. The complete blood count was performed before donation, and data relative to hemoglobin, leukocyte, and platelet values were obtained. A structured questionnaire was applied to all participants to collect information relative to risk factors for HEV infection. All the participants were informed about the purpose of the study.
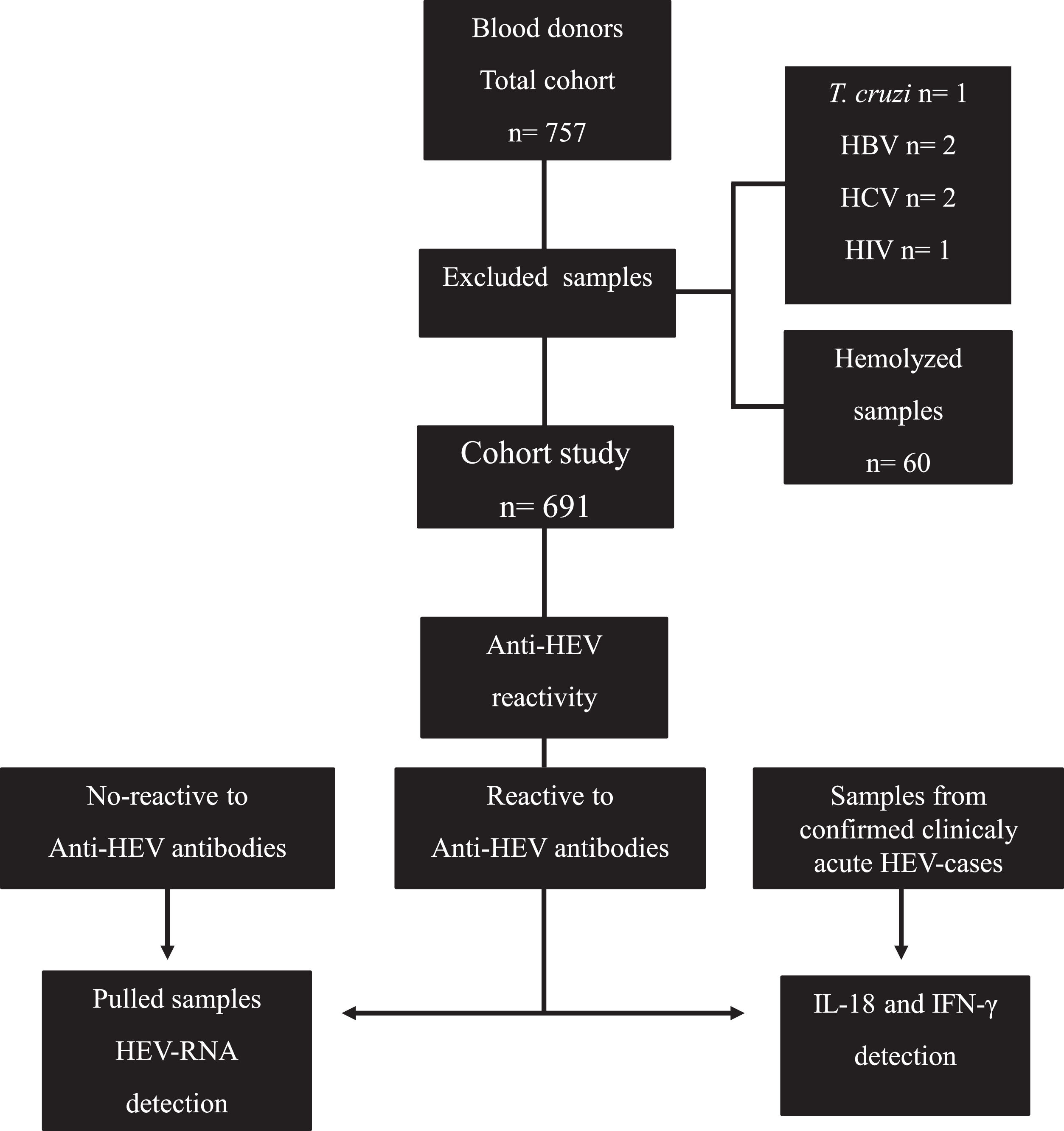
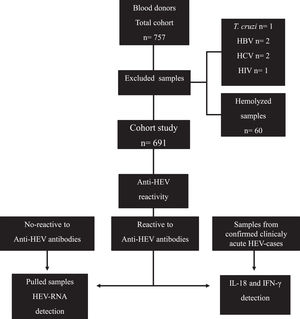
According to the analysis of blood bank records, out of the 757 candidates who donated blood and were included in this study, 6 were positive for pathogens (Trypanosoma cruzi, HBV, HCV and Human Immunodeficiency Virus [HIV]) tested as part of the blood donation protocol. These samples were discarded from our study. Of 751 remaining sera, 60 samples were hemolyzed, for which they were discarded; therefore, our total cohort study included 691 serum samples (Fig. 1).
Flow chart of the study. A total of 757 samples from blood donors were collected, of which 691 were included to identify reactivity to anti-HEV IgM/IgG. Sixty samples from seronegative donors, 60 samples from anti-HEV IgM/IgG seropositive donors, and 60 samples from clinically acute HEV patients were then screened for IL-18 and IFN-γ detection. The viral genome was investigated in 60 pooled available positive samples for anti-HEV immunoglobulins.
Serum samples were screened for anti-HEV immunoglobulin (Ig)G and anti-HEV IgM using a commercial enzyme-linked immunosorbent assay (ELISA) kit; 5 μL of donor serum was used, and tests were conducted following the manufacturer´s guidelines (MIKROGEN, Neuried, Germany). These tests are reported to have a sensitivity and specificity of 98.5% and 98.8% for IgG and 98.9% for IgM. Previously reported seropositive samples identified as clinically confirmed HEV patients were included as controls [11]. The final readout was obtained with a WHYM201 Microplate reader (Poweam Medical Co., Ltd, Nanjing, China).
2.4Serum pooling, RNA extraction and nested polymerase chain reaction for HEV RNA detectionThe viral genome was investigated in 60 pooled available positive samples for immunoglobulins anti-HEV (including either IgG and IgM/IgG single- or double-positive sera) in ten pools of six and in 60 pooled randomly selected seronegative samples in ten pools of six. Serum samples of HEV-seropositive donors were pooled by mixing 50 μL of each sample to reach a final volume of 300 μL. Pools were mixed homogeneously by pipetting several times and kept at room temperature until processing. A serum sample positive for HEV detection previously reported [11] was included. RNA extraction was performed from 300 μL of serum pools of donors and a 1:100 dilution of the positive sample with a QIAmp Viral RNA mini kit (QIAGEN, USA) according to the manufacturer's specifications. Reverse transcription was immediately conducted with 5 μL of RNA. HEV genome detection was performed by targeting the ORF-2/3 overlapping region to amplify 164 bp in the first round and 137 bp in the second round as previously described [12]. For Retro-transcriptase nested-PCR (RT-nPCR), 5 μL of purified RNA was used to carry out a two-step 12 μL reaction by using 1.75 μM primer for each reaction, 870 μM dNTP MIX (Promega, WI), 3 μL of M-MLV 5 × Reaction Buffer (Promega, WI) (Tris–HCl pH 8.3 250 mM, KCl 375 mM, MgCl2 15 mM, and DTT 50 mM), and 8.5 U of M-MLV RT (Promega, WI) as indicated by the manufacturer.
The first round of nested PCR using forward 5´GCRGTGGTTTCTGGGGTGAC-3´ and reverse 5´-CTGGGMYTGGTCDCDCGCCAAG-3´ primers was carried out in a 20 μL mix volume containing 4 μL of complementary DNA, 1 × PCR buffer (10 mM Tris–HCl pH 8.8, 50 mM KCl), 0.625 mM MgCl2, 0.25 mM dNTP mix, and 2.5 U Taq Polymerase. In the second round, 2 μL of the first round of PCR was used for amplification. Primers forward 5´-GYTGATCTCTCAGCCCTTCGC-3´ and reverse -5´-GMYTGGTCDCGCCAAGHGGA-3´ under the same cycling conditions as in the first step. PCR was carried out as previously described.
2.5IL-18 and IFN-γ detectionPositive serum samples for anti-HEV immunoglobulins (n=60) were further selected for the analysis of IL-18 and IFN-γ by ELISA (MBL, LTD, Beijing, China and Abcam, UK, respectively). Serum from donors negative for anti-HEV immunoglobulins (n=60) and 60 serum samples from confirmed cases of clinically acute hepatitis E previously reported were included as controls [11,20]. Samples were diluted 1:5 and tested according to the manufacturer´s instructions. The final readout was obtained with a WHYM201 Microplate reader (Poweam Medical Co., Ltd, Nanjing).
2.6Statistical analysesStatistical comparisons were performed by using GraphPad Prism software version 5.01 (GraphPad Software, Inc., San Diego, CA). All data were tabulated in Excel spreadsheets to calculate frequencies. The chi-square test was calculated for qualitative variables. For analysis of cytokine values, one-way ANOVA was used to calculate the statistical significance, and post hoc tests were used to identify differences between groups. Risk factor data are shown as median and intraquartile range, and differences between groups were traced using the Mann‒Whitney U parametric test. A P value of less than 0.05 was considered statistically significant.
2.7Ethical statementsWritten informed consent was obtained from each patient included in the study, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Local Committee of Investigation in Health Number 1305 at the IMSS R-2019-1305-059 and the Mexican Regulatory Agency COFEPRIS (17 CI 14 039 030).
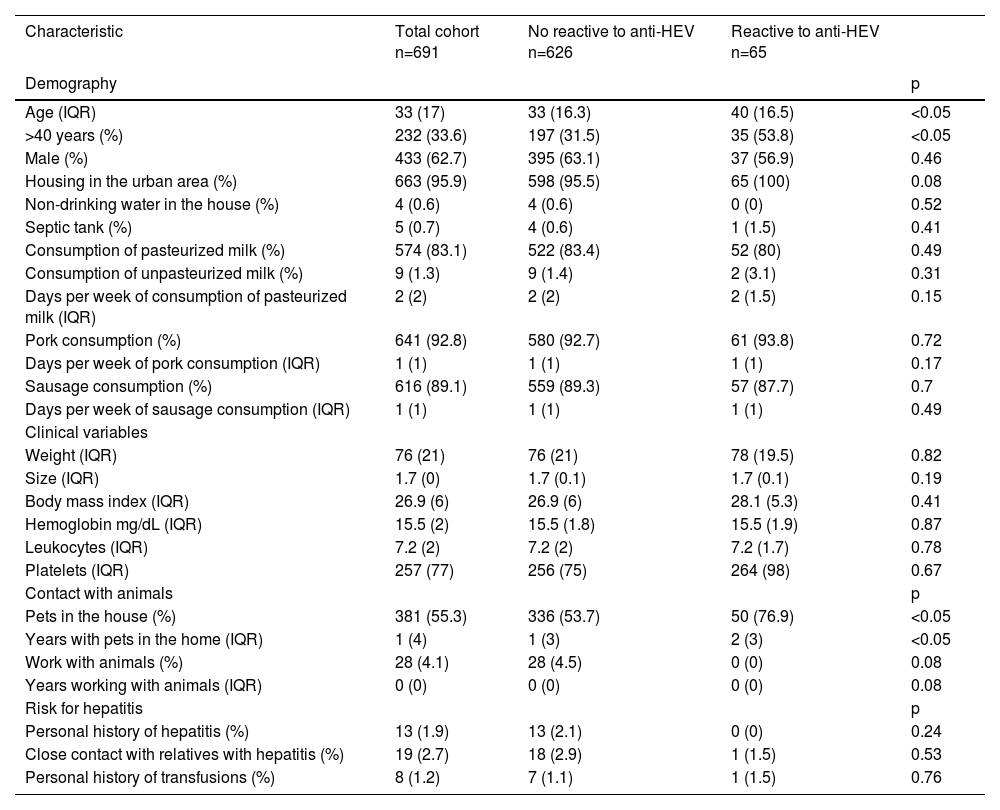
3Results3.1High frequency of anti-HEV antibodies in blood donorsOf our cohort, the median age was 33 years, and a third of the donors were older than 40 years; housing in the urban area was common for most of the individuals included, who were mostly men (Table 1). Of the 691 serum samples tested, 9.4% were reactive for antibodies against HEV by ELISA, either IgG, IgM or both. Of the total 65 positive samples, most of the individuals were positive only for IgG anti-HEV antibodies, and single-positive IgM anti-HEV samples were found in a lower proportion (Table 2).
HEV seroprevalence and risk factors for infection in blood donors.
| Characteristic | Total cohort n=691 | No reactive to anti-HEV n=626 | Reactive to anti-HEV n=65 | |
|---|---|---|---|---|
| Demography | p | |||
| Age (IQR) | 33 (17) | 33 (16.3) | 40 (16.5) | <0.05 |
| >40 years (%) | 232 (33.6) | 197 (31.5) | 35 (53.8) | <0.05 |
| Male (%) | 433 (62.7) | 395 (63.1) | 37 (56.9) | 0.46 |
| Housing in the urban area (%) | 663 (95.9) | 598 (95.5) | 65 (100) | 0.08 |
| Non-drinking water in the house (%) | 4 (0.6) | 4 (0.6) | 0 (0) | 0.52 |
| Septic tank (%) | 5 (0.7) | 4 (0.6) | 1 (1.5) | 0.41 |
| Consumption of pasteurized milk (%) | 574 (83.1) | 522 (83.4) | 52 (80) | 0.49 |
| Consumption of unpasteurized milk (%) | 9 (1.3) | 9 (1.4) | 2 (3.1) | 0.31 |
| Days per week of consumption of pasteurized milk (IQR) | 2 (2) | 2 (2) | 2 (1.5) | 0.15 |
| Pork consumption (%) | 641 (92.8) | 580 (92.7) | 61 (93.8) | 0.72 |
| Days per week of pork consumption (IQR) | 1 (1) | 1 (1) | 1 (1) | 0.17 |
| Sausage consumption (%) | 616 (89.1) | 559 (89.3) | 57 (87.7) | 0.7 |
| Days per week of sausage consumption (IQR) | 1 (1) | 1 (1) | 1 (1) | 0.49 |
| Clinical variables | ||||
| Weight (IQR) | 76 (21) | 76 (21) | 78 (19.5) | 0.82 |
| Size (IQR) | 1.7 (0) | 1.7 (0.1) | 1.7 (0.1) | 0.19 |
| Body mass index (IQR) | 26.9 (6) | 26.9 (6) | 28.1 (5.3) | 0.41 |
| Hemoglobin mg/dL (IQR) | 15.5 (2) | 15.5 (1.8) | 15.5 (1.9) | 0.87 |
| Leukocytes (IQR) | 7.2 (2) | 7.2 (2) | 7.2 (1.7) | 0.78 |
| Platelets (IQR) | 257 (77) | 256 (75) | 264 (98) | 0.67 |
| Contact with animals | p | |||
| Pets in the house (%) | 381 (55.3) | 336 (53.7) | 50 (76.9) | <0.05 |
| Years with pets in the home (IQR) | 1 (4) | 1 (3) | 2 (3) | <0.05 |
| Work with animals (%) | 28 (4.1) | 28 (4.5) | 0 (0) | 0.08 |
| Years working with animals (IQR) | 0 (0) | 0 (0) | 0 (0) | 0.08 |
| Risk for hepatitis | p | |||
| Personal history of hepatitis (%) | 13 (1.9) | 13 (2.1) | 0 (0) | 0.24 |
| Close contact with relatives with hepatitis (%) | 19 (2.7) | 18 (2.9) | 1 (1.5) | 0.53 |
| Personal history of transfusions (%) | 8 (1.2) | 7 (1.1) | 1 (1.5) | 0.76 |
Regarding demographic characteristics, seropositive cases, including IgG, IgM or both anti-HEV antibodies, were compared to seronegative cases, and seropositive cases were significantly older. Additionally, the majority of individuals with a positive serum sample were older than 40 years. The predominant living condition in positive samples was housing in the urban area, and all the individuals with a positive sample were living in that condition; this was not a statistically significant factor for positiveness. Living was consistent with the fact that no differences were found when housing conditions and feeding habits (the intake of pasteurized and nonpasteurized milk, rich diet in pork) were analyzed. Interestingly, most individuals positive for HEV reported having contact with pets, with a median of contact of 2 years, and the presence of pets at home was significantly higher in seropositive individuals.
None of the confirmed cases with positive serology reported having a personal history of hepatitis at some point in their lives. No statistically significant differences in the values of hemoglobin, leukocytes, and platelets from the complete blood were found between seropositive and seronegative data from donors (Table 1).
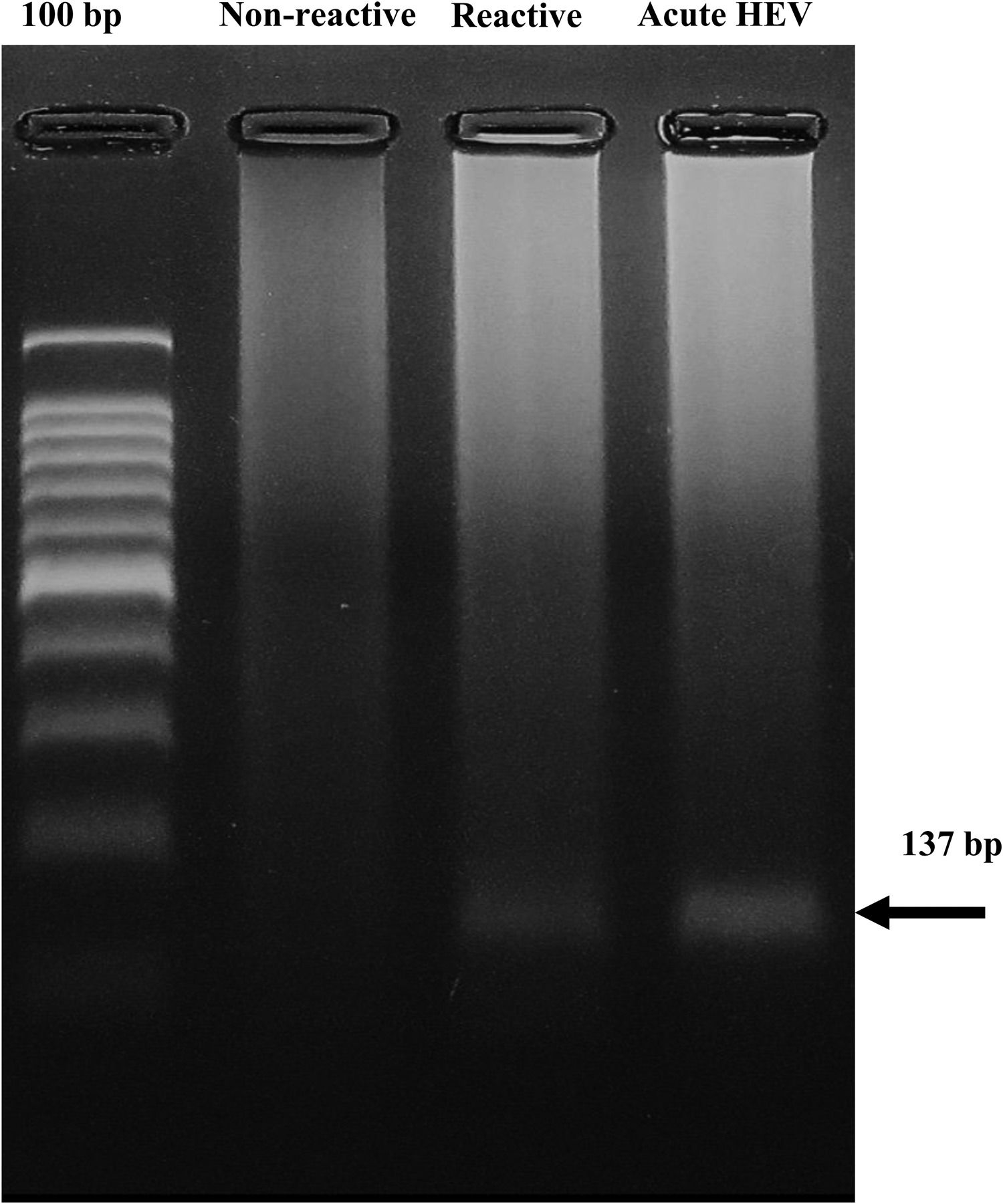
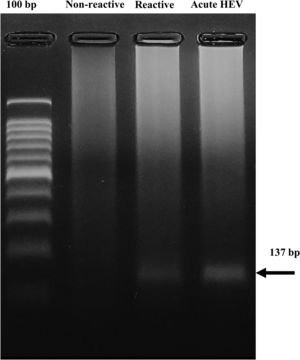
3.3HEV-RNA detection in blood donorsThe nested RT‒PCR method provided the 137-bp expected product in one of the 10 pools positive for anti-HEV immunoglobulins. No HEV genome was found in the pooled seronegative samples (Fig. 2).
Detection of HEV RNA in serum from blood donors reactive to anti-HEV antibodies. The 137 bp product found in one pool of six seropositive samples (Reactive) and in the sample from a confirmed HEV patient (acute HEV) is shown. Data from a representative pool of randomly selected seronegative samples for anti-HEV antibodies (not reactive) are shown.
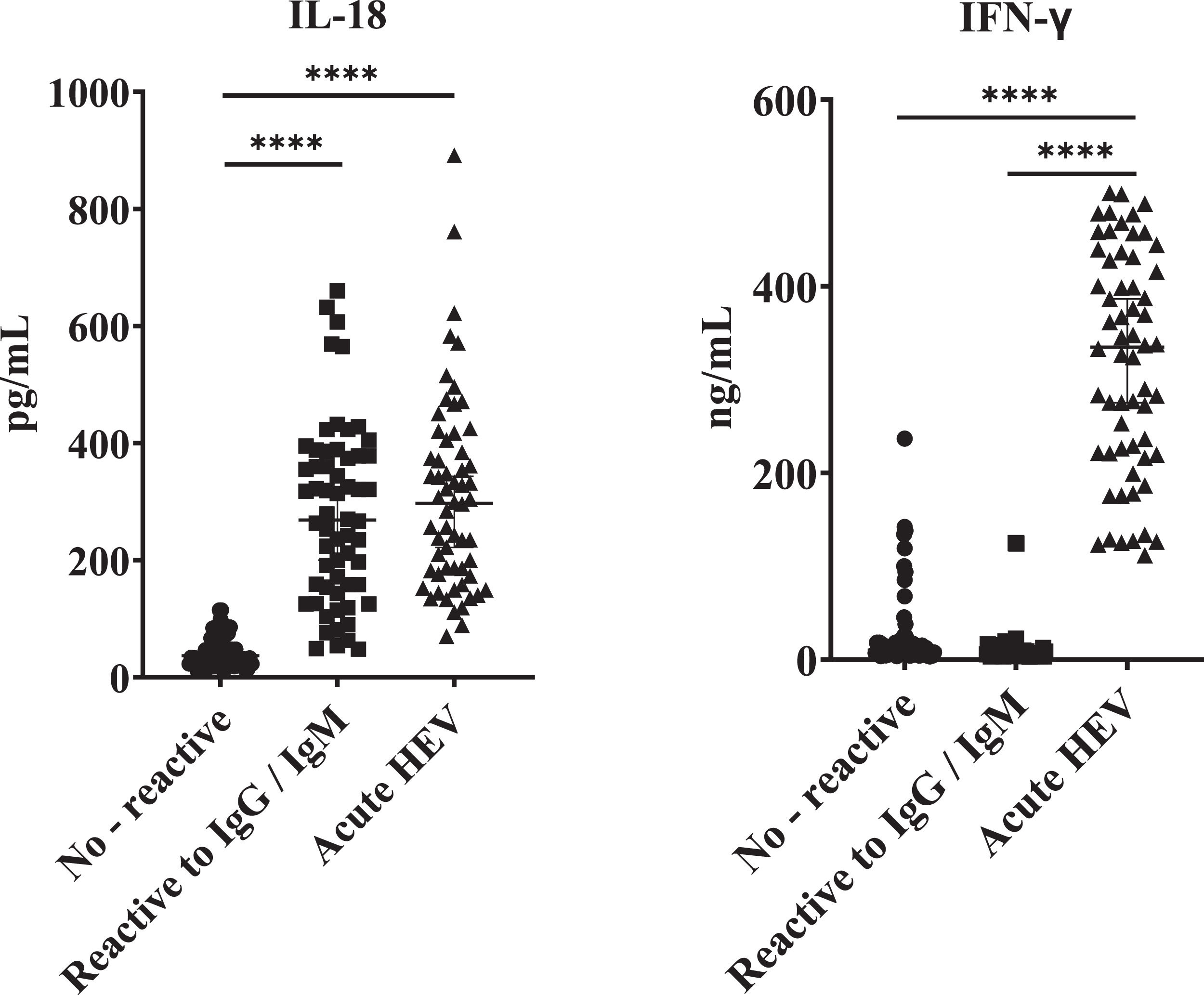
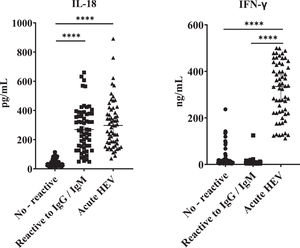
All the HEV seropositive samples of blood donors tested showed significantly high IL-18 concentrations relative to samples from seronegative donors. Interestingly, these values were similar when compared to samples from clinically acute confirmed HEV patients (Fig. 3). However, as we found for previously described variables, no significant differences were found by comparing IgM/IgG relative to IgG reactive donors in terms of IL-18 titers. Likewise, when analyzing the IFN-γ values, differences were found only when comparing the samples of patients with clinically acute HEV infection with the IgM/IgG anti-HEV seronegative and seropositive groups, and no differences in values of this cytokine between seronegative and seropositive groups were found.
IL-18 and IFN-γ concentrations in acute HEV patients and blood donors reactive and not reactive to anti-HEV antibodies. ELISA was performed to determine the concentration of IL-18 and IFN-γ in serum samples positive for immunoglobulins anti-HEV: not reactive (n=60), IgG/IgM anti-HEV reactive (n=60). Serum from donors negative for anti-HEV immunoglobulins (n=60) and serum samples from confirmed cases of acute hepatitis E (n=60) previously reported were included. Values ± the standard deviation are presented. P < 0.05 was considered statistically significant. ****P < 0.0001.
Efforts in HEV research in industrialized countries have driven the implementation of HEV screening in human blood derivatives in diverse regions; however, studies in middle- and low-income regions are still limited. The results of this study support the notion that HEV is a neglected disease in Mexico and underscore risk factors for infection and IL-18 as a potential biomarker of virus exposure.
Considering the sociodemographic characteristics of the people of Mexico and the mechanisms of HEV transmission, the seroprevalence obtained in the study carried out in Brazil in 2017 was used as a reference (9.8 in a total of 500 sera from healthy donors) [21]. Both the number of samples analyzed, and the prevalence reported in our study are very similar to the cited report and coincide with a recently reported cohort of 400 sera from blood donors from Uruguay [22]. Similar studies have been carried out in other regions of the world, reporting different prevalence of antibodies [23–26].
In relation to the risk factors for exposure to HEV, the evidence dictates that it is related to increasing age [27], as is the case in our study, where it was found that subjects over 40 years of age have a higher risk of exposure to the virus (OR 2.75 CI 95% 1.63-4.65, data not shown). The consumption of meat foods, mainly derived from pork, is most frequently related to the risk of exposure to HEV [28], but in our study, no relationship was found between this factor and reactivity to the ELISA test. These data may be related to the fact that most of the analyzed sera in our study were from individuals from urban areas. In fact, a high HEV seroprevalence has previously been reported in individuals from a rural population in northern Mexico [29]. Therefore, specific studies in distinct regions of Mexico should be conducted to determine the real picture of HEV infection. No relationship was found between the consumption of pasteurized milk and the seroprevalence of HEV. Likewise, close contact with animals has been identified as a risk factor for HEV infection, either at work or at home [28]. Interestingly, our data underscore that the presence of pets in the home increases the risk of exposure to HEV by 3.19 (95% CI 1.74-5.84) times.
Changes in IL-18 concentration in serum have been associated with prognosis in viral infections, such as dengue virus and SARS-CoV-2 [30,31]. The role of IL-18 during hepatitis E has not been elucidated. However, diverse models of acute HEV infection indicate an increase in the concentration of IL-18 in serum [32]. Herein, a significant increase in IL-18 concentration was found in seropositive individuals relative to blood donors nonreactive for HEV serology. Surprisingly, the values we found in the seropositive group were similar to the values of IL-18 present in the serum of patients with confirmed acute HEV infection. In contrast, a significant increase in IFN-γ levels was found only in sera from clinically acute confirmed HEV patients relative to seropositive and seronegative samples from blood donors. Importantly, the evidence of a small fragment of HEV RNA detection supports the notion of acute infection in at least one of the donors in our study. This result is especially relevant considering the recognized difficulty in detecting the HEV genome in blood donors worldwide [33] and highlights that blood banks may be a source of virus transmission in Mexico.
The finding of IFN-γ in confirmed acutely HEV-infected patients coincides with the recognized antiviral effect of IFN-γ during the acute phase of viral diseases, whereas activation of the inflammasome complex resulting from previous exposure to HEV [32], might be responsible for IL-18 upregulation we found in anti-HEV seropositive individuals. Therefore, in addition to reactivity for HEV serology and viral RNA detection, cytokines might be useful to delimitate the status of HEV infectivity and, their study represents a field for future investigation.
Finally, the increased IL-18 levels we found in individuals with previous exposure to HEV is consistent with the upregulated expression of this cytokine in viral diseases including HCV and HIV infections [16,17] and suggest that IL-18 may represent a bona fide biomarker for diverse viral etiologies including those related to liver disease. Considering that hepatic function is not routinely tested in blood donors, future studies are necessary to delimitate the potential use of IL-18 screening to distinguish exposure to viral agents affecting liver function in blood banks.
5ConclusionsAltogether, our findings highlight the imperative need to follow up on the presence of HEV in blood banks in low- and middle-income regions, especially in those where the virus burden is still not well known. In this sense, IL-18 could represent a biomarker of HEV exposure in blood donors. A systematic study and deeper knowledge of HEV, including HEV screening in the recipients of blood derivatives, is needed to determine the transmission mechanism. The finding that the presence of pets in the home increases the risk of exposure to HEV supports that virus sources need to be revisited to effectively cut the transmission chain. Altogether, these findings might be extended to other regions, and will enable clinicians to better handle this commonly underdiagnosed disease.
FundingThis work was partially funded by DGAPA-UNAM Grant IA201422 to NAF.
CRediT authorship contribution statementEdgar D. Copado-Villagrana: Methodology, Data curation, Formal analysis, Writing – original draft. Antonio Pizuorno: Conceptualization, Writing – original draft. Adrián García-Suárez: Methodology. Julio C. Abarca: Methodology. Gisela DuPont: Project administration. Socorro Jaramillo-Bueno: Data curation. Nora A. Fierro: Conceptualization, Funding acquisition, Supervision, Writing – original draft.
The authors thank Edmundo Lamoyi and Javier Orozco-Córdova for critical reading of the manuscript.