Breast and non-small cell lung cancers harbor an upregulated CSNK2A2 oncogene that encodes the protein kinase CK2 alpha', a catalytic subunit of the highly conserved serine/threonine kinase CK2. However, its role and biological significance in hepatocellular carcinoma (HCC) remains unclear.
Materials and MethodsWestern-blotting and immunohistochemistry were used to measure the expression of CSNK2A2 in HCC tumor tissues and cell lines. CCK8, Hoechst staining, transwell, tube formation assay in vitro and nude mice experiments in vivo were used to measure the effects of CSNK2A2 on HCC proliferation, apoptosis, metastasis, angiogenesis and tumor formation.
ResultsIn the study, we showed that CSNK2A2 was highly expressed in HCC comparison with matched control tissues, and was linked with lower survival of patients. Additional experiments indicated that silencing of CSNK2A2 promoted HCC cell apoptosis, while inhibited HCC cells migrating, proliferating, angiogenesis both in vitro and in vivo. These effects were also accompanied by reduced expression of NF-κB target genes, including CCND1, MMP9 and VEGF. Moreover, treatment with PDTC counteracted the promotional effects of CSNK2A2 on HCC cells.
ConclusionsOverall, our results suggested that CSNK2A2 could promote HCC progression by activating the NF-κB pathway, and this could serve as a biomarker for future prognostic and therapeutic applications.
During the past few decades, the yearly incidence and mortality rate of liver cancer has been on the rise, causing it to become the third major cause of cancer-related deaths around the world [1]. The most common primary liver cancer is hepatocellular carcinoma (HCC) and it is commonly associated with chronic conditions such as hepatitis B (HBV) and hepatitis C (HCV) infection [2,3]. More recently, it has been recognized that non-alcoholic fatty liver disease (NAFLD) represents a previously ignored factor for HCC [4]. Despite new emerging treatments for reducing the incidence of liver cancer, most patients are still associated with poor prognoses. Thus, an understanding of the clinical, pathological and molecular characteristics of HCC would be useful to potentially identify or develop more effective therapeutic targets against HCC.
CK2, a highly conserved and ubiquitous serine/threonine protein kinase, consists of two catalytic subunits namely CK2α, CK2α', as well as two regulatory ones (CK2β) [5]. As a multifunctional and ubiquitously expressed protein kinase, CK2 can individually catalyze the phosphorylation of a significant fraction of eukaryotic proteome, with most studies supporting its role in triggering the phosphorylation of key protein substrates or as a priming protein kinase for hierarchical phosphorylation signaling. However, recent studies have also found that CK2 is abnormally expressed in high levels in different types of cancers, including liver, lung, breast, prostate, colon as well as head and neck cancers [6–12]. In addition, highly-expressed CK2 is not only linked to clinicopathological parameters but also promotes the migration, proliferation and epithelial-to-mesenchymal transition (EMT) of tumor cells by regulating the stability of IκBs and promyelocytic leukemia protein (PML) as well as the phosphorylation of IKK, p65 and snail, with these features causing the kinase to be considered as a ‘‘hallmark of cancer’’[9,13–17].
IκBs, IKK and p65 are all integrated within the NF-κB pathway. First identified in 1986 as a nuclear transcription factor, NF-κB acts by binding to the enhancer region that regulates the expression of the light chain of activated B cell immunoglobulin κ A [18]. As a heterologous or homologous dimeric complex consisting of NF-κB1(P50), NF-κB2(P52), RelA(P65), RelB and C-REL, NF-κB has been identified in breast, colon, lung, and ovarian epithelial tumors [19] and given that it is activated in almost all tumor tissues, it is not surprising that this complex promotes tumor cell proliferation, metastasis, EMT and angiogenesis. Although in most cases, NF-κB activation is inhibited in the cytoplasm by specific IκBs inhibitor, this pathway can be activated in three ways. The first two, referred to as the classic NF-κB activation pathway and the non-classical NF-κB activation pathway, depend on the IKKα dimer and the phosphorylation of the IκB kinase complex (IKKα/β/γ), while the third one, known as the atypical pathway, is triggered by DNA damage (e.g., UV radiation) or doxorubicin. In this case, the activation of CK2 phosphorylation is induced by p38 before subsequently mediating IκBα ubiquitination and degradation for activating the NF-κB pathway [20]. Interestingly, many studies have also reported that CK2 could even mediate IKKs and P65 phosphorylation, thereby suggesting that it could probably be involved in the classic and non-classical NF-κB activation pathways as well [9,12,15,16]. Hence, it appears that CK2 can also act as a priming protein kinase for NF-κB phosphorylation signaling. Some previous studies further reported that the overexpression of CK2 can promote tumor proliferation and metastasis by upregulating the expressions of VEGF, IL8 and other angiogenesis markers [12]. Besides, studies have shown that CK2α and CK2β could encourage hepatocellular carcinoma proliferation, metastasis and angiogenesis via the activation of the NF-κB pathway [6,21], while CK2α' can drive lung cancer metastasis by regulating BRMS1 [8]. However, since the role of CK2α' (CSNK2A2) in the progression of HCC is yet to be reported, this work investigated the expression levels and biological functions of CSNK2A2 in HCC.
2Materials and Methods2.1Collection of sample tissuesSampling was performed on primary HCC patients at the Hunan Provincial People's Hospital of Changsha, China. Thirty pairs of HCC tissues, along with matched adjacent normal ones, were collected and immediately immersed in RIPA lysis buffer before being frozen at -80°C until processed for protein extraction. For immunohistochemical (IHC) assays, an additional primary HCC sample was first fixed with 4% paraformaldehyde and after being embedded in paraffin, several sections of 5-μm thickness were cut for IHC staining. This experiment was carried out with the approval of the Ethics Committee of the Hunan Provincial People's Hospital. All the patients also understood and signed the informed consent.
2.2Cell culture, proliferation and apoptosis assayHuman liver cell lines, namely Bel-7402, Huh7, HepG2, Bel-7404 and MHCC97H, were provided by the Gene Function Laboratory of Hunan Normal University of Changsha, China. Cell cultures were established in DMEM (High Glucose) medium containing 10% Fetal Bovine Serum (FBS, Biological Industries) and 1% penicillin/streptomycin (Hyclone) before being incubated at 37 °C and under 5% CO2. Fresh medium was added every two days to maintain growth and once the cells had reached around 90% confluency, they were detached using Trypsin-EDTA (0.05%), with the collected cells subsequently used for assays. For proliferation assays, Huh7 and MHCC97H liver cell lines were detached and resuspended in DMEM medium to obtain a concentration of 1 × 105 cells/ml. 100μl of the cell suspension were first seeded into 96-well plates and 24 h later the cell counting kit-8 solution (MCE, China; 10 μl/well) was added. This was followed by a 4-h incubation at 37 °C and under 5% CO2 before eventually using a microplate reader (Biotek) to read absorbance values at 450 nm. In addition, apoptosis assays were also performed with Hoechst 33258 (sigma) staining. For this purpose, Huh7 and MHCC97H liver cell lines were seeded onto sterile glass coverslips for cell fixing using 4% paraformaldehyde. After washing the coverslips with 1 × PBS, the Hoechst 33258 solution was then applied for cell staining and left for 10 min. The coverslips were finally inverted onto clean glass slides for viewing under a fluorescence microscope (Lecia).
2.3Transwell migration assayThis assay was carried out using an 8-μm transwell apparatus (Millipore). Briefly, Huh7 and MHCC97H liver cell lines were detached and resuspended in DMEM medium to obtain a concentration of 1 × 105 cells/ml. To the upper and lower chambers of the transwell apparatus, 200 μl of the cell suspension and 600 μl of medium, supplemented with 10% FBS, were then respectively added. After 16 h, the chambers were immersed for 30 min in 4% paraformaldehyde prior to 30-min staining using 1% crystal violet. Finally, after washing the chambers with 1 × PBS, the unmigrated cells were removed from the inner chambers with the help of cotton swabs while those which had migrated were photographed with the help of an inverted microscope (Carl Zeiss).
2.4Endothelial tube formation assayBD Matrigel Basement Membrane matrix was thawed on ice and added into a pre-cooled 48-well plate which was then kept for 1 h at 37 °C in a 5% CO2 incubator. To this Matrigel-coated plate, HUVEC cells were detached and resuspended in EBM2 media (with 5% FBS) at a density of 2 × 104 cells/ml, 200 μl of HUVEC cells was added and after a 16-h incubation. Endothelial tube formation was observed under an inverted microscope (Carl Zeiss). Analysis of results was performed with the NIH image J software to determine the number of branches.
2.5Western blot analysisHCC tissues and human liver cell lines were lysed in RIPA buffer for total protein extraction. The resulting proteins were loaded onto SDS-PAGE, and after separation, they were transferred to polyvinylidene fluoride membranes (Millipore) which were then blocked with 5% Bovine Serum Albumin (BSA) before being incubated with the following primary antibodies: Anti-CSNK2A2 (abcam, ab10474), CCND1 (abcam, ab16663), VEGF (proteintech, 19003-1-AP), MMP9 (proteintech, 10375-2-AP), Phospho-NF-κB p65 (Ser536) (Cell Signaling Technology, 3033T), NF-κB p65 (Cell Signaling Technology, 8242T) and GAPDH (proteintech, 60004-1-Ig). After overnight incubation with the antibodies, the membranes were washed and a second incubation with HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H+L) (proteintech, SA00001-2) or HRP-conjugated Affinipure Goat Anti-Mouse IgG (H+L) (proteintech, SA00001-1) was performed for 1 h. ECL Western Blotting Substrate (Solarbio, PE0010) was eventually used to visualize the results with the help of the Bio-Rad ChemiDoc XRS+ imaging system.
2.6Quantitative real-time analysisHuman liver cell lines were harvested for total RNA extraction using the AG RNAex Pro Reagent (Accurate Biology, AG21101). After reverse transcription of the extracted RNA (1 μg) using the Evo M-MLV RT Premix (Accurate Biology, AG11706), the resulting cDNA was amplified by quantitative real-time PCR (qPCR) on an ABI Q3 system by using the SYBR® Green Premix Pro Taq HS qPCR Kit (Accurate Biology, AG11701). For the qPCR, the primer sequences for CSNK2A2 were 5′-TCCCGAGCTGGGGTAATCAA-3′ (Forward) and 5′-GTTCCACCACGAAGGTTCTCC-3′ (Reverse) while in the case of GAPDH, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and 5′-GGCTGTTGTCATACTTCTCATGG-3′ were used as the forward and reverse primers respectively. The relative transcript level of CSNK2A2 was eventually determined based on the 2−△△CT method.
2.7Immunohistochemical (IHC) analysisThe collected tissue sections (HCC and matched adjacent normal ones) first underwent the process of dewaxing, rehydration, antigen retrieval. Blocking was then achieved at room temperature using 5% BSA for 1 h prior to overnight incubation with CSNK2A2 antibodies. On the following day, the sections were washed two times with 1 × TTBS (5 min each) and were then incubated with the horseradish peroxidase-conjugated secondary antibody. After staining with DAB substrate, the sections were eventually sealed with neutral resin and visualized under an inverted microscope (Carl Zeiss). For this experiment, the IHC scores were based on both the percentage of stained cells and the signal intensity. More specifically, for the percentage, a score of 0, 1, 2 or 3 was given when <10%, 10-25%, 25-50% and >50% of cells were stained respectively, while for the signal intensity, scores were given according to the following: 0 in the absence of staining; 1 if a light brown color was observed; 2 if the color was brown; and 3 for dark brown [22].
2.8Animal experimentsFour- to six-weeks old BALB/c male nude mice were housed in the SPF animal laboratory of the Hunan Provincial People's Hospital where, along with 12-h light/12-h dark cycles, they were given adequate water and food. Huh7-shCSNK2A2, Huh7-shControl, MHCC97H-shCSNK2A2 and MHCC97H-shControl stable cell lines were constructed by infecting with shControl and shCSNK2A2 lentiviral particles and then selected with 4 μg/ml puroMycin (Gibco). Tumorigenesis experiments were then performed by subcutaneous injections of Huh7-shCSNK2A2, MHCC97H-shCSNK2A2 as well as control cell lines into the left or right flanks of the mice. The length and width of the tumors were analyzed as described in a previous study [21]. All animal care experiments were reviewed and approved by the Ethics Committee of the Hunan Provincial People's Hospital.
2.9Statistical analysisStatistical analyses were performed using SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism (version 8.0, GraphPad Software). Results obtained from repeated experiments (triplicates) were used to present the data as means ± standard error of means (SEM) while additional analyses involved one-way analysis of variance (ANOVA), Dunnett's post hoc test for multiple group comparison, as well as pairwise group comparisons using Student's t-tests. In this case, differences with *P < 0.05, **P < 0.01, ***P < 0.001 were considered to be statistically significant. Finally, using the Kaplan-Meier method, survival curves were plotted before being compared using log-rank tests.
2.10Ethical statementsIn the study, written informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee of Hunan Provincial People's Hospital (Ethics Section 2021 No. (Hunan 30)).
All animal experiments were conducted in accordance with the ARRIVE guidelines and carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments, or the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
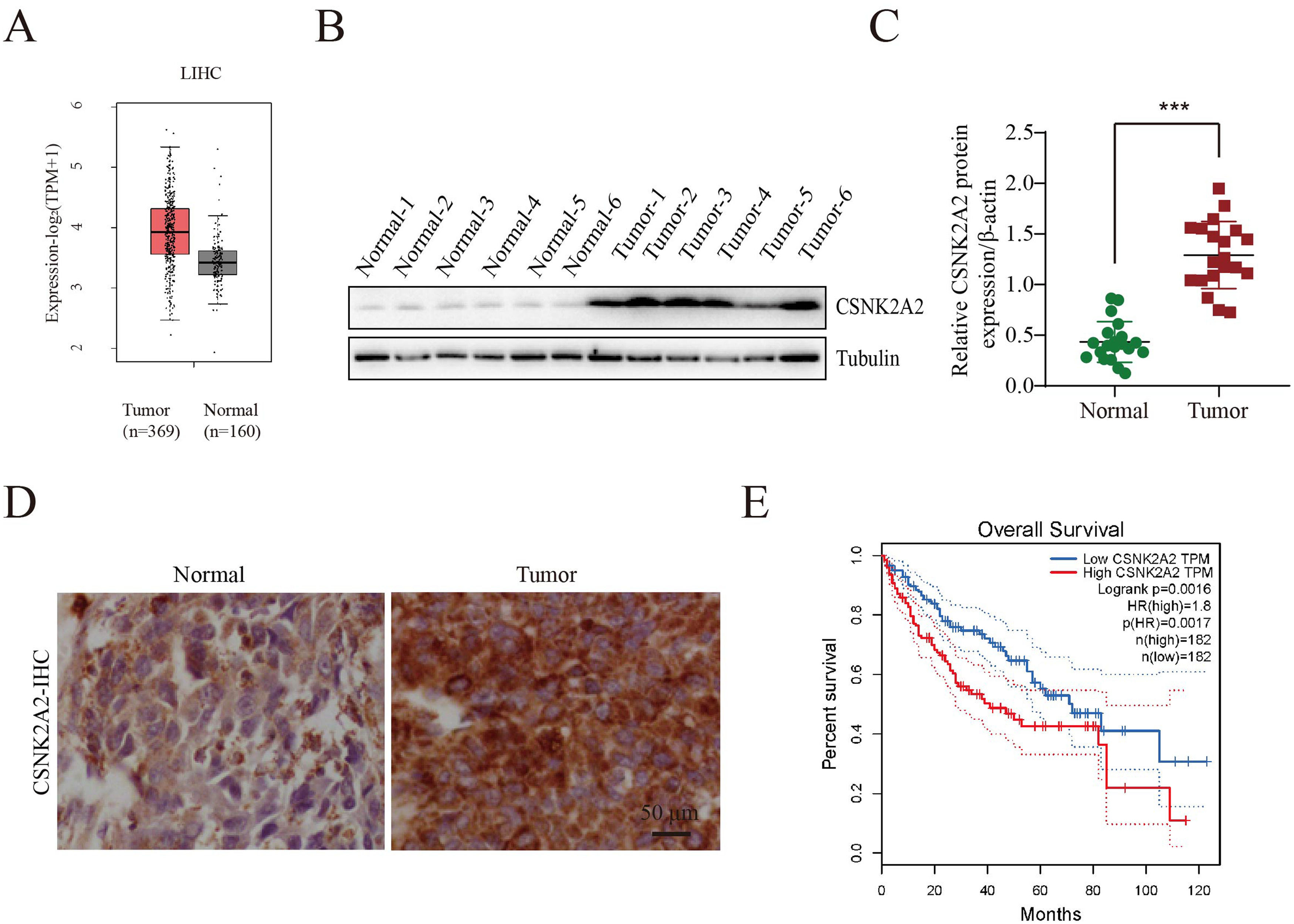
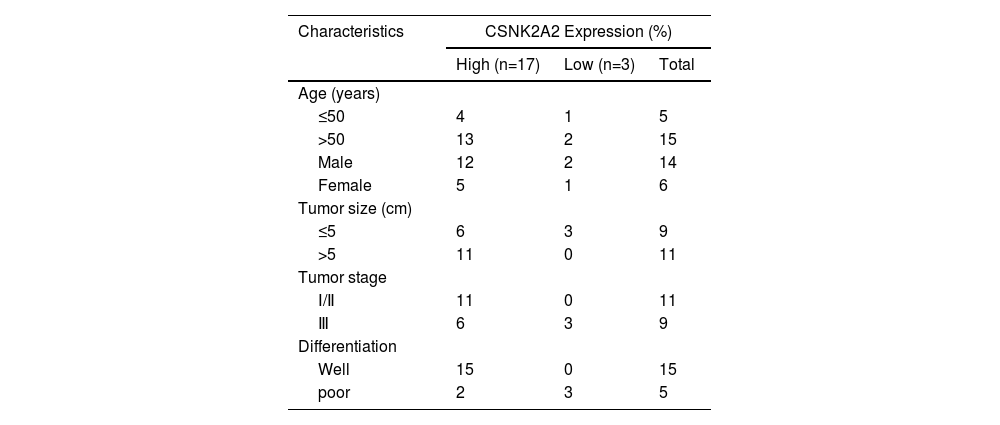
3Results3.1Upregulation of CSNK2A2 in HCC tissues and its association with patient prognosisIt has been reported that, compared with adjacent noncancerous tissues, there was an overexpression of the protein kinase CK2 alpha' gene (CSNK2A2), encoding a catalytic subunit of CK2, in both human non-small cell lung cancer (NSCLC) and neck squamous cell carcinoma (HNSCC). However, since CSNK2A2 expression in HCC is yet to be reported, this work examined the gene's expression profile in this type of cancer through the use of the Gene Expression Profiling Interactive Analysis (GEPIA) web server. The results showed a significantly higher expression of CSNK2A2 in tumor samples (n=369) compared with normal ones (n=160) (Fig. 1 A). Similarly, analysis of western blots (Fig. 1B and 1C) indicated that levels of the CSNK2A2 protein in cancerous tissues from the 20 HCC patients were higher than for the matched adjacent normal ones while in the case of IHC assays, tumors exhibited stronger CSNK2A2 intensity than primary tumor tissues (Fig. 1D). Clinicopathological association analyses of the 20 HCCs revealed that CSNK2A2 expression was significantly associated with tumor size, tumor stage and tumor differentiation (Table 1). Finally, a survival analysis using an online Kaplan-Meier Plotter database was performed to examine whether there was a link between the expression level of CSNK2A2 and the clinical prognosis of HCC patients, with the results, shown in Fig. 1E, indicating that lower survival was significantly associated with high expression of CSNK2A2.
Increased expression of CSNK2A2 in HCC tissues and its ability to predict prognosis.
Oncomine-based analysis of CSNK2A2 expression in HCC patients (n=369) and normal samples (n=160) in TCGA dataset. B-C. CSNK2A2 expression levels in HCC tumor and peritumor samples was determined by western blotting. D. Micrographs of HCC tissues showing high and low CSNK2A2 expression levels. E. Survival of HCC patients having high or low CSNK2A2 Transcripts per million (TPM) based on the Kaplan-Meier method. Cancers with high CSNK2A2 TPM (n=182) are represented by red lines while blue ones represent cancers with low CSNK2A2 TPM (n=182). Statistical significance was determined using the log-rank test.
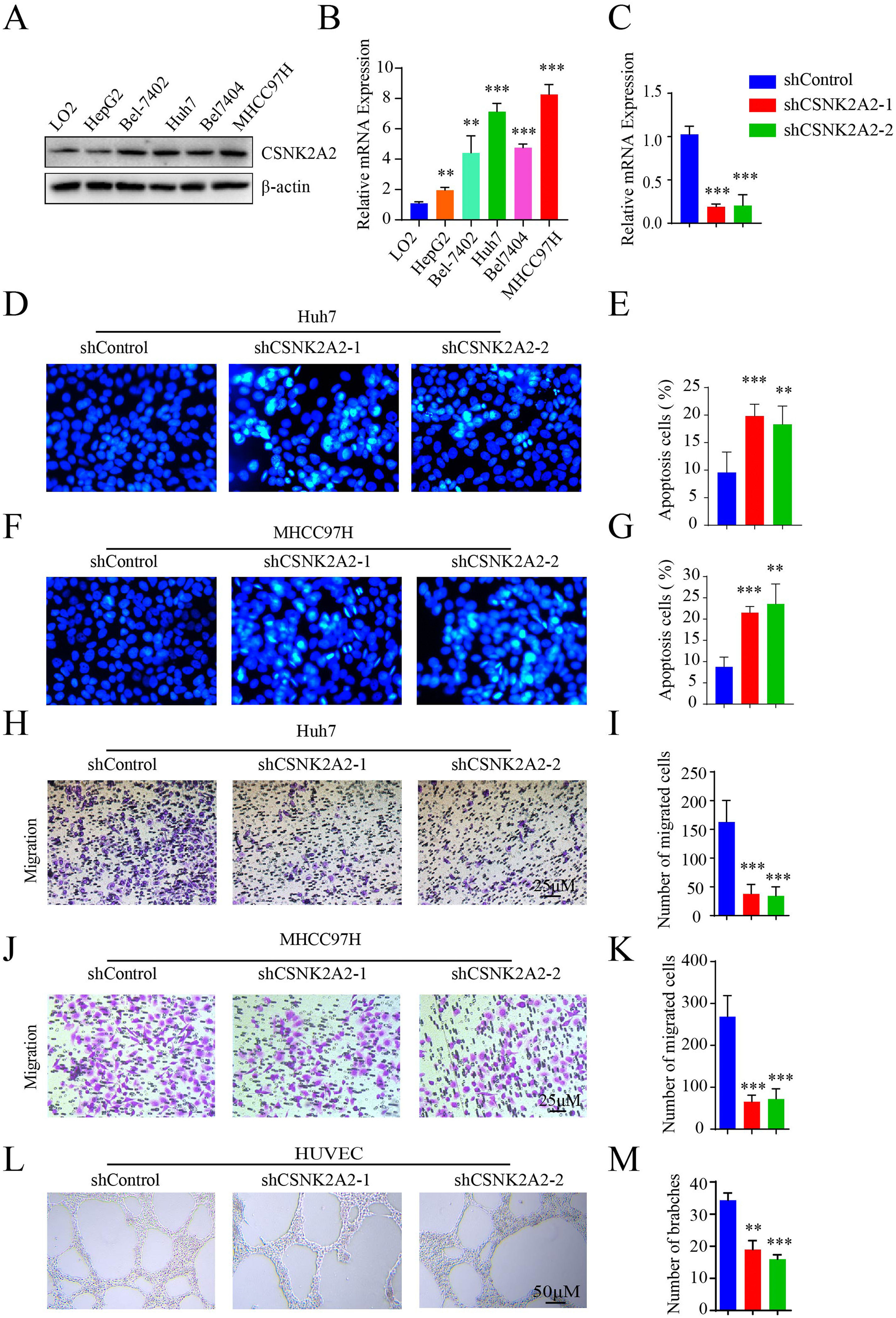
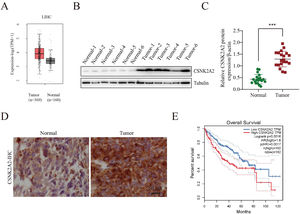
Based on the above results regarding the upregulation of CSNK2A2 and its correlation with poor prognosis, it was speculated that this gene could be involved in HCC progression. To investigate this feature, levels of the CSNK2A2 protein in HepG2, Bel-7402, Huh7, Bel-7404 and MHCC97H cell strains as well as non-malignant cells (LO2) were first measured. In this case, the protein levels in HCC cell lines were higher than for non-malignant ones (Fig. 2A), with qPCR also confirming these results (Fig. 2B). Furthermore, the biological functions of CSNK2A2 in HCC were also determined by knocking down the gene in Huh7 and MHCC97H cell lines which could highly express CSNK2A2. This was achieved by infecting the cells with lentiviruses expressing shCSNK2A2, and as shown in Fig. 2C, mRNA levels were successfully reduced in both cell lines. In terms of the gene functions, it was previously reported the use of TGF-β1 to inhibit CK2 activity could promote HCC cell apoptosis [23], thereby indicating that silencing CSNK2A2 could cause HCC cell apoptosis. These effects were, therefore, investigated based on Hoechst 33258 staining, with the results confirming the presence of a greater number of typical morphologically-distinct apoptotic bodies in both Huh7-shCSNK2A2 and MHCC97H-shCSNK2A2 cell lines compared with the control (Fig. 2D–2G). In addition, many studies not only suggest that tumor metastasis is either considered as the terminal step of tumor progression or the initial stage of further metastasis but also report that the knockdown of CSNK2A1 and CSNK2B could inhibit the metastasis of many tumor cells. In this context, the results showed that the degree of cell migration for Huh7 and MHCC97H indeed decreased after gene silencing compared with the control group (Fig. 2H–K). Finally, it is also recognized that angiogenesis is critical for tumor cells metastasis and proliferation and in order to investigate whether CSNK2A2 could regulate angiogenesis-induced HCC progression, HUVECs cells were infected with shCSNK2A2-expressing lentiviruses. In this case, it was observed that those cells harboring the silenced gene had a significantly lower number of branch points than the control (Fig. 2L and 2M). Altogether, the above results highlight the fact that silencing CSNK2A2 could repress HCC cells migration and endothelial tube formation while promoting apoptosis.
CSNK2A2 promotes HCC cell migration and endothelial tube formation.
A-B. mRNA levels of CSNK2A2 as determined by western blotting and qPCR as well as differences in protein expression between HCC cell lines (HepG2, Bel-7402, Huh7, Bel-7404 and MHCC97H) and a normal hepatocyte (**P<0.01, ***P<0.001, one-way ANOVA). C. mRNA levels of CSNK2A2 in Huh7 cells infected with shCSNK2A2 or shControl lentiviruses as determined by qPCR (***P<0.001, one-wayANOVA). D-E. Hoechst staining to test and quantify cell apoptosis in Huh7 cells which had been transformed using lentiviruses that expressed shCSNK2A2 or shControl (**P<0.01, ***P<0.001, Student's t-test). F-G. Hoechst staining to test and quantify apoptosis in MHCC97H cells which had been transformed with lentiviruses that expressed shCSNK2A2 or shControl (**P<0.01, ***P<0.001, Student's t-test). H-I. Migration results for Huh7 cells which had been transformed using lentiviruses that expressed shCSNK2A2 or shControl (***P<0.001, Student's t-test). J-K. Migration results for MHCC97H cells which had been transformed using lentiviruses that expressed shCSNK2A2 or shControl (***P<0.001, Student's t-test). L-M. Results of endothelial tube formation for HUVEC cells which had been transformed using lentiviruses that expressed shCSNK2A2 or shControl (**P<0.01, ***P<0.001, Student's t-test).
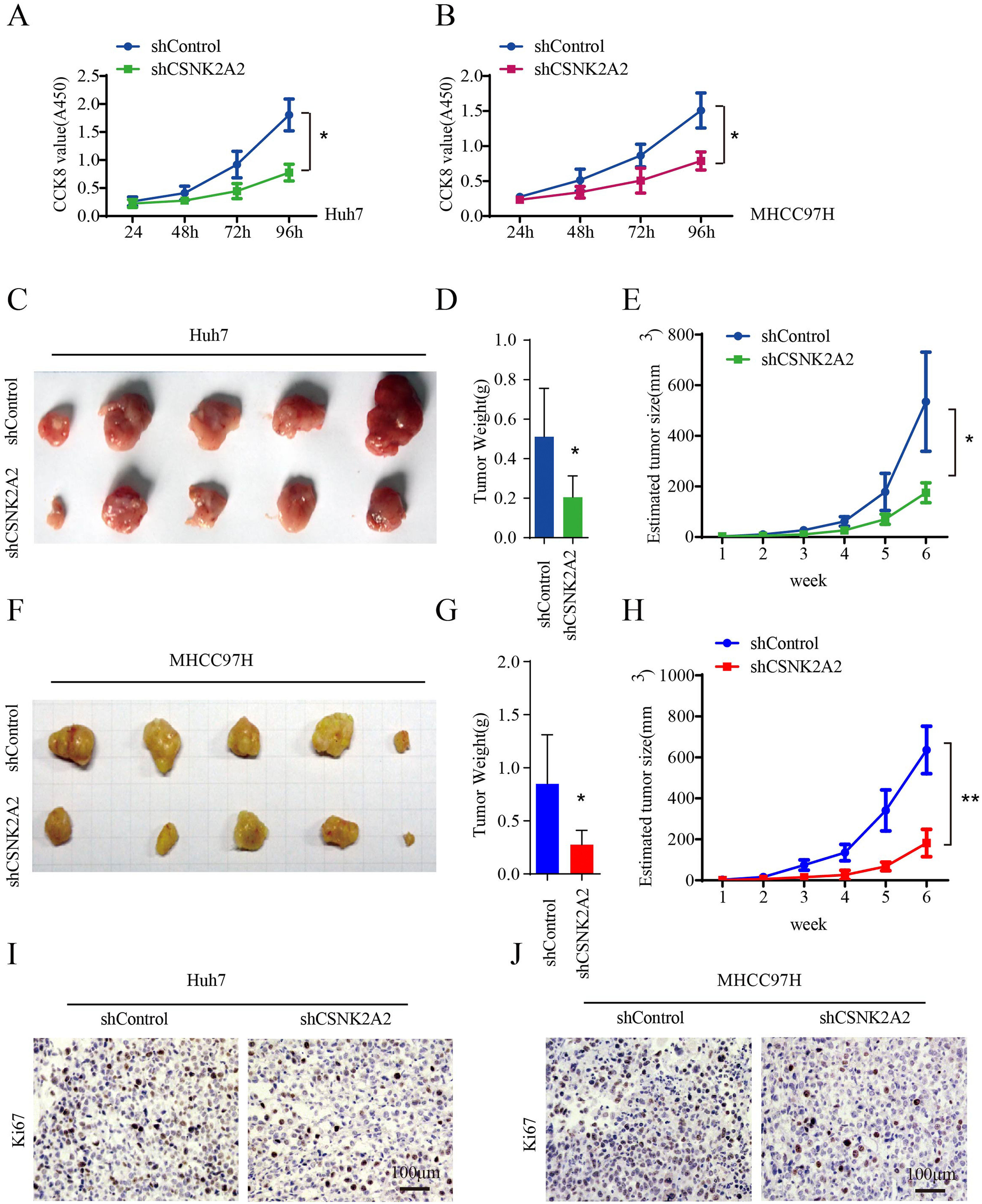
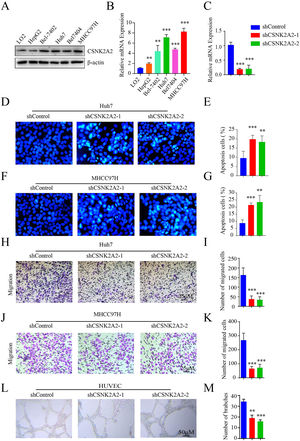
Many studies have reported that the overexpression of CSNK2A1 and CSNK2B promoted HCC cell proliferation [21,24]. In this study, the vitality of Huh7 and MHCC97H cell lines was lower after being infected with lentiviruses expressing shCSNK2A2 (Fig. 3A and 3B), thus suggesting that the knockdown of CSNK2A2 could repress HCC cell proliferation. To further examine the effects of CSNK2A2 on the in vivo cell growth of HCC, nude mice were subcutaneously injected with Huh7 and MHCC97H cell lines as well as control ones. Weekly measurements of the tumor sizes were then taken while the tumor weights were determined on the sixth week. The results indicated that tumor volumes and tumor weights, derived from Huh7-shCSNK2A2 and MHCC97H-shCSNK2A2 cell lines, were respectively smaller and lighter compared with those obtained from CSNK2A2-silenced controls (Fig. 3C–3H). These results were further confirmed when sections of the xenografted tumors, induced by Huh7-shCSNK2A2 and MHCC97H-shCSNK2A2 cell lines, were stained with ki67 antibody. In this case, it was found that the percentage of positive ki67 staining was lower in cells for which CSNK2A2 was silenced (Fig. 3I and 3J). Taken together, these data supported the fact that the silencing of CSNK2A2 could significantly repress the proliferation of HCC cells along with the growth of xenografted tumors.
CSNK2A2 regulates HCC cell proliferation in vitro and vivo.
A. CCK8 was used to test Huh7 cell proliferation after infection with lentiviruses that expressed shCSNK2A2 or shControl (*P<0.05, Student's t-test). B. Results of CCK8 tests to determine MHCC97H cell proliferation after infection with lentiviruses that expressed shCSNK2A2 or shControl (*P<0.05, Student's t-test). C. Tumors after injecting Huh7-shCSNK2A2 or Huh7-shControl cell lines for six weeks. D-E. Quantification of tumor volume and weight in nude mice after injecting Huh7-shCSNK2A2 or Huh7-shControl cell lines for six weeks (*P<0.05, Student's t-test). F. Tumors after injecting MHCC97H-shCSNK2A2 or MHCC97H-shControl cell lines for six weeks. G-H. Quantification of tumor volume and weight in the mice after injecting MHCC97H-shCSNK2A2 or MHCC97H-shControl cell lines for six weeks (*P<0.05, Student's t-test). I. Anti-Ki67-based staining of xenografted tumors from Huh7-shCSNK2A2 or Huh7-shControl, scale bar, 100 μm. J. Anti-Ki67-based staining of xenografted tumors from MHCC97H-shCSNK2A2 or MHCC97H-shControl, scale bar, 100 μm.
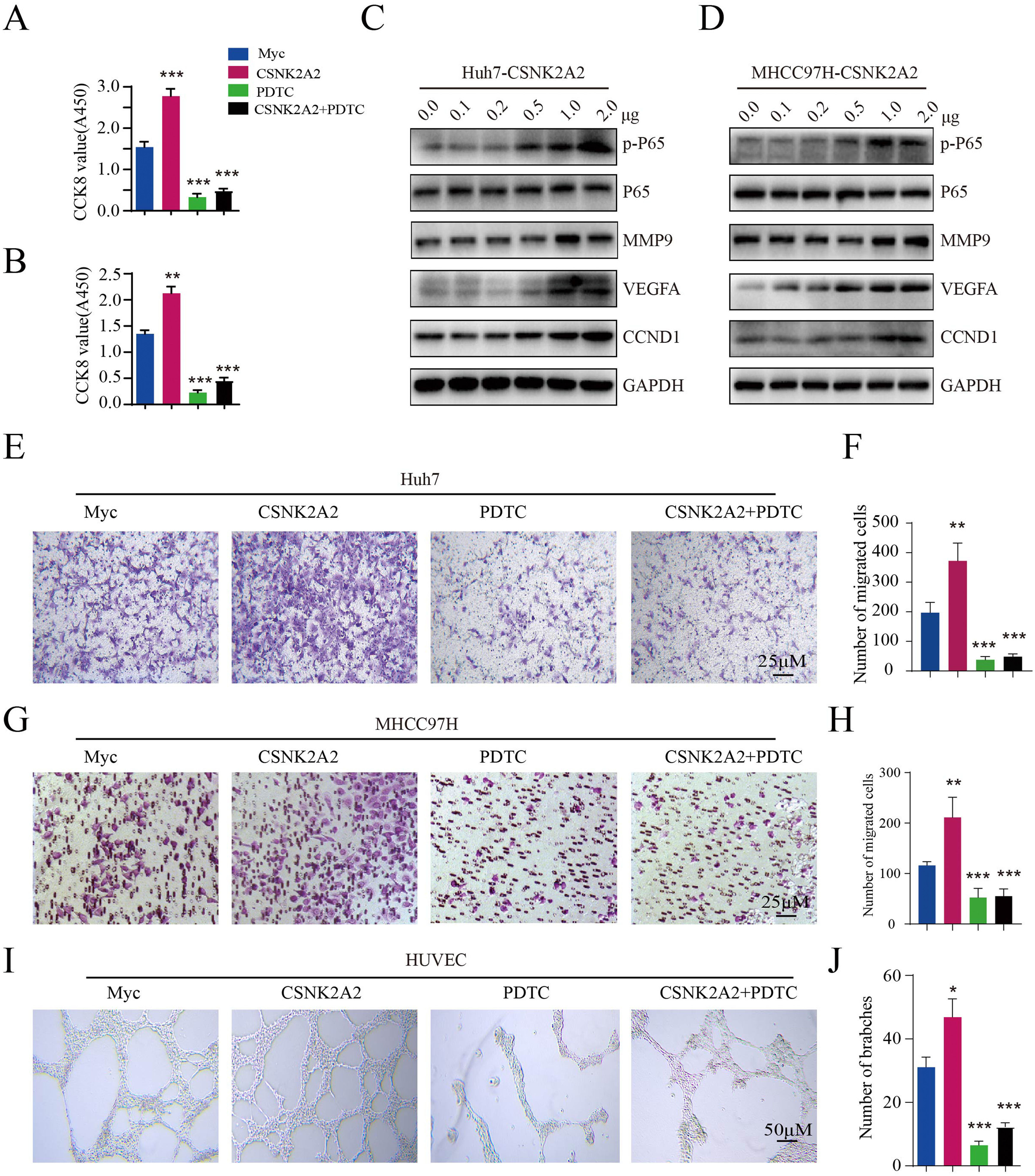
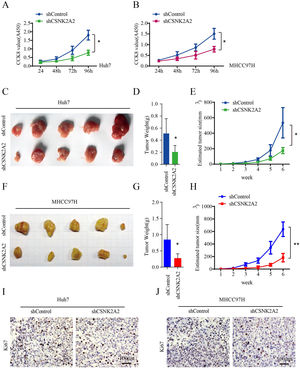
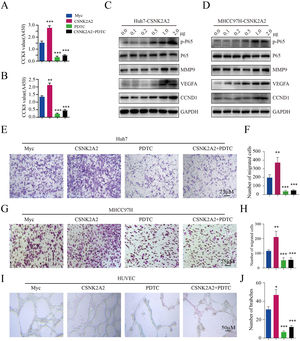
Existing evidence points out that CSNK2A1 and CSNK2B phosphorylates IKKα/β/γ and p65 subunit and induces Phospho-NF-κB p65 into nuclear, then activates NF-κB pathway. To investigate whether CSNK2A2 can activate the NF-κB pathway, CSNK2A2-containing plasmids were transfected into Huh7 and MHCC97H cells. Based on western blot analyses, it was found that CSNK2A2 enhanced phospho-p65 levels, while also upregulating the expression of NF-κB target genes (MMP9, VEGF and CCND1) (Fig. 4A and 4B), thus indicating that the overexpression of CSNK2A2 could activate the NF-κB pathway. To further investigate whether CSNK2A2 could promote HCC cell proliferation, metastasis and endothelial tube formation by activating this pathway, CSNK2A2-containing plasmids as well as control ones were transfected into Huh7 and MHCC97H cells, prior to treatment with pyrrolidine dithiocarbamic acid (PDTC) which specifically blocks the NF-κB pathway. As shown by the results of CCK8 (Fig. 4C and 4D) and trans-well (Fig. 4E–4H) analyses, the overexpression of CSNK2A2 promoted the migration and proliferation of both Huh7 and MHCC97H cells, but similar effects were not observed after treatment with PDTC. Similarly, while the number of branches for HUVEC cells, indicated by the results of the tube formation assay (Fig. 4I and 4J), increased after being transfected with CSNK2A2-containing plasmids, no such effects were obtained when cells were treated with PDTC. Altogether, these findings indicated that CSNK2A2 could accelerate HCC progression by activating the NF-κB pathway.
Activation of the NF-κB pathway by CSNK2A2, along with the upregulation of genes involved in HCC migration, proliferation and endothelial tube formation.
A. CCND1, MMP9, VEGF, p65 and p-p65 expression in Huh7 cells which had been transformed using lentiviruses that expressed shCSNK2A2 or shControl. Expression levels were determined by western blotting, with β-actin used as the control. B. CCND1, MMP9, VEGF, p65 and p-p65 expression in MHCC97H cells which had been transformed using lentiviruses that expressed shCSNK2A2 or shControl. Expression levels were determined by western blotting, with β-actin used as the control. C. CCK8 was used for testing Huh7 cell proliferation after infection with shCSNK2A2 and subsequent treatment with 20 μM of PDTC (***P<0.001, Student's t-test). D. CCK8 was used for testing MHCC97H cell proliferation after infection with shCSNK2A2 and subsequent treatment with 20 μM of PDTC (**P<0.01, ***P<0.001, Student's t-test). E-F. Migration results for Huh7 cells infected with shCSNK2A2 before treatment with 20 μM of PDTC (**P<0.01, Student's t-test). G-H. Migration results for MHCC97H cells infected with shCSNK2A2 before treatment with 20 μM of PDTC (**P<0.01, Student's t-test). I-J. Results of endothelial tube formation for HUVEC cells infected with shCSNK2A2 before treatment with 20 μM of PDTC (*P<0.05, ***P<0.001, Student's t-test).
The present study demonstrated the functions of CSNK2A2 (CK2α') in promoting cell apoptosis, migration, proliferation and tube formation in HCC. Previous studies on CK2 have mainly considered the roles of CK2α and CK2β, with comparatively less work focused on those of CK2α'. It has been confirmed that the three subunits of CK2 are highly expressed in several cancers, leading to increased levels of CK2 that are tightly correlated with poor prognosis. Hence, as expected, the current work confirmed the upregulation of CK2α' in HCC tissues as well as its tight link with patient prognosis. In addition, when knocking down the gene for CK2α', HCC cell proliferation, migration and endothelial tube formation were repressed, apoptosis was promoted and the growth of tumors in vivo was blocked. Thus, it was a clear indication that CK2α' could contribute to HCC progression.
Reports of a previous study suggest that the inhibition of CK2α could promote HCC cell apoptosis after treatment with TGF-β1 by decreasing levels of the CK2α' protein [23] but whether this downregulation blocked IκBα phosphorylation for inducing HCC cell apoptosis was not supported. Nevertheless, as illustrated in Fig. 2D–2G, the results from knockdown experiments supported the fact that CK2α’ could indeed induce HCC cell apoptosis. These were consistent with studies reporting significant decreases in the expression of anti-apoptotic genes (BCL2 and BCL-XL) as well as increased expression of proapoptotic ones (TP53 and TAp63) when the effects of CK2α' on head and neck cancer were studied [25]. Besides, it was also found that silencing the CK2α′ gene could repress HCC cell proliferation while effectively blocking the growth of tumors in vivo. In this context, a number of studies have already confirmed that the overexpression of CK2α and CK2β could promote cancer cell proliferation in bladder cancer, prostate cancer, breast cancer and HCC [21,26-28]. On the other hand, as far as CK2α′ was concerned, while one study has shown that its inhibition could suppress glioblastoma (GBM) cell survival after infection with CK2a' siRNAs [29], another one found no effects of CK2α′ knockdown on the proliferation of head and neck cancers [25]. Therefore, the results not only indicate that overexpression of CK2α′ could promote HCC cell proliferation but also suggest that different CK2 subunits may contribute to cell proliferation through different means.
It is well documented that the knockdown of the CK2α gene could inhibit the migration and invasion of most cancer cells including colorectal cancer, prostate cancer, bladder cancer, head and neck cancer and HCC [6,25-27,30-32]. In contrast, the knockdown of the CK2β gene appeared to have opposing effects in head and neck cancer as well as normal breast epithelial cells [25,33]. However, as far as this study was concerned, the results indicated that the knockdown of CK2α′ gene could significantly block HCC cell migration (Fig. 2H–2K) and these were consistent with its reported effects on lung cancer [8] even though no similar observations were made for the in vitro cell migration of head and neck cancer cells [25]. In this context, it should be remembered that EMT (epithelial-to-mesenchymal transition) is regarded as essential for mediating cancer cell migration, invasion, and metastasis, with CK2 playing a vital role in the process [34]. Interestingly, the CK2α catalytic subunit and CK2β regulatory subunit show an oppose role to the biology progress. On the one hand, knockdown of CK2α was recently linked with poor ability of EMT. On the other hand, a dysregulation of CK2β expression reversely contributes to EMT by stabilizing snail and maintaining the mesenchymal phenotype [17,33,35]. However, it is worth noting that since the role of CK2α′ on EMT is not yet well understood, it would be interesting to explore the biological functions of CK2α′ on EMT as part of future investigations.
Tumor metastasis involves communication between different tissues and cells, with angiogenesis being important for this complex process [36]. Inhibition of CK2 activity has been reported as repressing angiogenesis both in vivo and in vitro[37–39]. At the same time, as illustrated in Fig. 2L, 2M as well as Fig. 4I, 4J, the present findings supported the fact that the knockdown of CK2α′ could decrease angiogenesis in HUVECs, while its overexpression had opposite effects through the regulation of VEGF expression. Targeting angiogenesis is regarded as the main form of treatment for HCC [40] and this is often the mode of action of currently-used drugs such as sorafenib, cabozantinib, ramucirumab and lenvatinib which act by inhibiting the activation of VEGFR [40]. Similarly, CX4945, as a potent and specific inhibitor of CK2α and CK2α', exhibits antitumor efficacy by inhibiting pro-survival and angiogenic signaling. Thus, exploring potentially effective inhibitors of CK2α′ has been proposed as a possible strategy for HCC therapy.
5ConclusionsThis work identified CK2α′ as a critical regulator of HCC progression. In particular, it can promote HCC proliferation, metastasis and angiogenesis while promoting the expression of genes involved in the processes (CCND1, MMP9 and VEGFA). Overall, it is expected that these findings regarding the functions of CK2α′ could be of potential importance for developing therapies against HCC.
FundingThis work was supported by the Natural Science Foundation of Hunan province (No. S2022JJQNJJ0430).
CRediT authorship contribution statementShuang Yang: Investigation, Project administration, Writing – original draft. Li Rong Peng: Funding acquisition, Data curation, Methodology. Ai Qing Yu: Data curation, Supervision. Jiang Li: Project administration, Supervision, Writing – review & editing.
The authors gratefully thank the Hunan Provincial People's Hospital for Hepatocellular Carcinoma tumor tissue and corresponding peritumor tissue collection.