Introduction. The progression of hepatic disease in chronic viral hepatitis is accompanied by an increased production of reactive oxygen species (ROS), as well as an accumulation of oxidative DNA damage, which is primarily repaired through base excision repair. XRCC1 (X-ray repair cross complementing protein 1) is one of the most important proteins involved in this repair pathway. The present study was carried out to verify the possible association of the XRCC1 rs25487 polymorphism with cirrhosis in patients from Central-West Brazil.
Material and methods. A total of 227 individuals with viral hepatitis, 53 cirrhotic and 174 non-cirrhotic, were genotyped for the XRCC1 rs25487 polymorphism using PCR-RFLP. Results: There were significantly higher frequencies of both the Arg/Gln genotype and of individuals with at least one Gln allele (Arg/ Gln+Gln/Gln) among cirrhotic patients (56.6% and 69.8%) compared with non-cirrhotic patients (25.8% and 37.9%). Both conditions were significantly associated with cirrhosis, independent of age, sex, alcohol intake or tobacco use (adjusted OR = 3.5, CI = 1.7–7.4, p = 0.001 and adjusted OR = 3.1, CI = 1.5–6.3, p = 0.002, respectively). Similar results were obtained for a group of HCV-infected patients but not for HBV-in-fected patients. Conclusions. The XRCC1 rs25487 polymorphism may influence the development of cirrhosis in viral hepatitis patients, and additional investigation will be necessary.
Chronic infections by the hepatitis B virus (HBV) and the hepatitis C virus (HCV) are major health problems that affect millions of people worldwide. In Brazil, it is estimated that there are 1.5 million HBV carriers and 2.5 million people infected with HCV.1
Persistent infections by HBV and HCV have variable outcomes ranging from minimal lesions throughout the liver parenchyma to severe fibrosis, cirrhosis and hepatocellular carcinoma (HCC).2 Cirrhosis occurs in 20–30% of chronically infected HBV patients and 10–15% of chronically infected HCV patients.3–5 It is an irreversible condition and is one of the main factors that predisposes patients to HCC. As a result, cirrhosis is considered a pre-malignant lesion.5–7
As a consequence of inflammatory cytokines, chronic inflammation generates reactive oxygen (ROS) and reactive nitrogen species that can damage several molecules, including DNA. The DNA damage triggers short-term responses, such as the activation of DNA repair, cell cycle checkpoints and, when the level of DNA damage exceeds the repair capacity, apoptosis. In the long-term, non-repaired DNA damage may result in mutations and higher genomic instability, which contribute to the development of chronic diseases and carcinogenesis.8–10
Base excision repair is the main repair pathway that protects cells against oxidative DNA lesions. XRRC1 (X-ray repair cross complementing protein 1), one of the most important molecules in base excision repair, forms a multi-protein complex with DNA ligase III, DNA polymerase β and Poly(ADP-ribose) polymerase to repair the single strand break generated by the cleavage of the DNA backbone at an abasic site, which occurred in an earlier step of the DNA repair mechanism.11,12
The role of DNA repair in liver function and homeostasis has recently been demonstrated in mice in which age-related liver dysfunction was accompanied by an accumulation of DNA damage.13 In viral hepatitis, both ROS and DNA damage have been connected with hepatic disease progression. An increase in ROS and DNA damage precedes the development of HCC in mice with active chronic hepatitis, which strongly indicates that both ROS and DNA damage are involved in carcinogenesis-related chronic infections.14 In addition, higher levels of oxidative DNA damage have been shown to correlate with the degree of hepatic lesions in HBV and HCV.15,16
The observation that not all chronically infected subjects progress to hepatic disease indicates that genetic factors also contribute to their clinical outcomes. Most genetic variability is conferred by single nucleotide polymorphisms. The XRCC1 rs25487, an A to G transition at codon 399 (exon 10) of the XRCC1 gene, results in a change from an arginine amino acid (Arg) to a glycine (Gln) within the XRCC1 protein. The activity of the protein can be altered by the presence of Gln, resulting in less effective DNA repair.17 There is also evidence to suggest that this polymorphism may have some influence on the susceptibility to hepatitis-related HCC and to chronic inflammatory diseases.18–20
Several reports suggest that DNA repair may play a pivotal role in the maintenance of normal liver function and that higher levels of DNA damage occur in the liver necroinflammatory process prior to the development of HCC related to viral hepatitis. Furthermore, a previous study completed with a Brazilian population suggests that this polymorphism increases the risk of developing alcoholic cirrhosis.21 However, few studies have investigated a possible association between the XRCC1 rs25487 polymorphism and the susceptibility to cirrhosis in chronic HBV and HBV infections. The present study was carried out to verify the hypothesized association between the XRCC1 rs25487 polymorphism and cirrhosis in Brazilian viral hepatitis carriers.
Material and MethodsStudy populationIn total, 227 patients with chronic viral hepatitis were investigated after recruitment from the Clinic of Infectious Diseases at Julio Muller Hospital (Federal University of Mato Grosso, Cuiabá, Mato Grosso State, Brazil). Chronic HBV infection was confirmed by persistent HBV surface antigenemia, which lasted more than six months. Chronic HCV infection was confirmed by the presence of HCV RNA in blood tests. Cirrhosis was diagnosed through a liver biopsy or through clinical, laboratorial and ultrasonographic evidence. As a control group, 176 healthy blood donors with a confirmed absence of infection were recruited from the Public Blood Bank of Mato Grosso State. Information regarding a donor’s alcohol consumption, tabagism, ethnicity and age was obtained from medical records and from an interviewer-administered questionnaire, which also included questions about the familial history of infectious diseases and cancer.
This study was approved by the Ethical Research Board at Julio Muller Hospital (protocol number 439/CEP-HUJM/07), and informed consent for voluntary participation was given by all participants.
GenotypingThe genomic DNA was isolated from 5 mL of peripheral blood collected in EDTA-vacutainers (Becton-Dickinson, Franklin Lakes, NJ) using a slightly modified salting-out assay.22 The genotyping was carried out using PCR-RFLP with the primers XRCC1 399F (5’-CCC CAA GTA CAG CCA GGT C-3’) and 399R (5’-TGC CCC GCT CCT CTG AGT AG-3’) (Integrated DNA Technologies, Belo Horizonte, MG).23 The PCR was performed in a total reaction volume of 20 μL that contained 20 mM Tris/HCl (pH 8.0), 50 mM KCl, 1.5 mM MgCl2, 0.5 μM of each primer, 0.2 mM dNTPs, 50 ng of genomic DNA and 1 U of Taq DNA polymerase (Invitrogen, Carlsbad, CA). The amplification conditions consisted of 5 min of an initial denaturation at 94 oC and 2 min of annealing at 58 °C, followed by 26–35 cycles of melting (94 oC for 45 s), annealing (58 oC for 45 s) and extension (72 oC for 54 s) and finished with an extension at 72 oC for 10 min. The amplicon was digested with the restriction enzyme MspI (New England Biolabs®, Ipswich, MA) at 37 °C overnight. The digestion products underwent an electrophoretic run at 250 V for 1 h and were visualized on a polyacrylamide gel with 10% silver nitrate staining. The wild-type Arg allele was identified by the presence of two bands (148 and 94 bp), and the Gln allele was identified by the presence of the uncut fragment (242 bp).
Statistical analysisThe age comparisons between groups were performed using the non-parametric Kruskal-Wallis test. The levels of transaminases in the patients of different genotypes were compared using the Mann-Whitney test. The Fisher’s exact test was used to compare polymorphism frequencies, as well as to compare ethnicity, sex, alcohol intake and tabagism between groups. To investigate the association of the XRRC1 polymorphism with disease or clinical manifestation, the odds ratios (OR) and respective 95% confidence intervals (95% CI) were calculated. A logistic regression model was constructed using Stata 8.2 (StataCorp, College Station, Texas, USA, 2005) to verify the independence of the XRCC1 genotypes from sex, age, alcohol intake and tabagism. The adherence of the genotypic distribution to the Hardy-Weinberg equilibrium was verified using the χ2 test. A value of p < 0.05 was used as the criterion of significance. The statistical analyses were performed using the BioEstat 5.0 statistical software (free software).
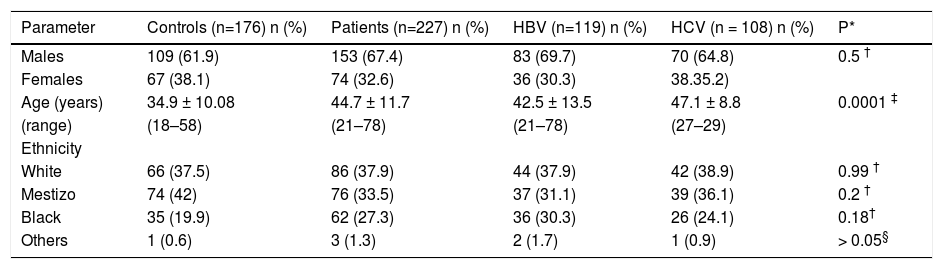
ResultsOf the 227 patients tested, 108 were HCV positive and 119 were HBV positive. The mean age of the control group (34.09 ± 10.1 years) was significantly lower than that of the other groups; the HCV-infected patients had a mean age of 47.1 ± 8.8 years, which was significantly higher than the HBV-infected patients (42.5 ± 13.5 years). The number of people of White, Black and Mestizo (people of mixed White, Black and Amerindian ethnicity) ethnicities was similar for all groups (Table 1). The alcohol consumption ranged from 0 to 8 g/day, with alcohol use being more frequently (p = 0.03) reported by the controls (33%) than by the patients (21%). Tobacco use was confirmed in 19.3% of the control population, 16.8% of the HBV-infected patients and 20.2% of the HCV-infected patients (p = 0.92).
Demographic characteristics of chronic HBV or HCV infected patients and controls subjects.
| Parameter | Controls (n=176) n (%) | Patients (n=227) n (%) | HBV (n=119) n (%) | HCV (n = 108) n (%) | P* |
|---|---|---|---|---|---|
| Males | 109 (61.9) | 153 (67.4) | 83 (69.7) | 70 (64.8) | 0.5 † |
| Females | 67 (38.1) | 74 (32.6) | 36 (30.3) | 38.35.2) | |
| Age (years) | 34.9 ± 10.08 | 44.7 ± 11.7 | 42.5 ± 13.5 | 47.1 ± 8.8 | 0.0001 ‡ |
| (range) | (18–58) | (21–78) | (21–78) | (27–29) | |
| Ethnicity | |||||
| White | 66 (37.5) | 86 (37.9) | 44 (37.9) | 42 (38.9) | 0.99 † |
| Mestizo | 74 (42) | 76 (33.5) | 37 (31.1) | 39 (36.1) | 0.2 † |
| Black | 35 (19.9) | 62 (27.3) | 36 (30.3) | 26 (24.1) | 0.18† |
| Others | 1 (0.6) | 3 (1.3) | 2 (1.7) | 1 (0.9) | > 0.05§ |
Others: Asian and Amerindian;
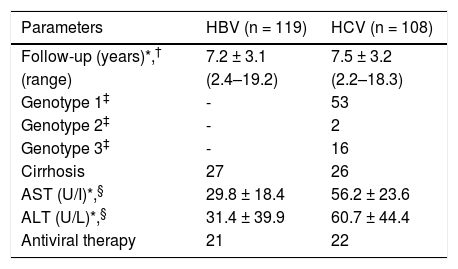
In total, 53 patients (23.3%) presented with cirrhosis, of whom 27 (22.1%) were HBV-infected and 26 (24.1%) were HCV-infected. There was not a significant difference in the percent of patients with cirrhosis between the groups (p = 0.74). The cirrhotic patients had a mean age that was significantly higher (p < 0.01) for both the HBV group (54.4 ± 13.2 years) and the HCV group (51.1 ± 8.7 years) compared to the non-cirrhotic patients (mean age 39 ± 11.5 and 46 ± 8.5 years, respectively). The HBV-infected patients presented levels of aspartate amino-transferase-AST (29.8 ± 18.4 U/L) and alanine aminotransferase-ALT (31.4 ± 23.6 U/L) within the values considered normal (AST: up 31 U/L for women and 37 U/L for men; ALT: up 31 U/L for women and 41 U/L for men). On the other hand, the HCV-infected patients had levels of AST and ALT that were higher than the normal values (56.2 ± 39.9 and 60.7 ± 44.4 U/L, respectively) (Table 2).
Clinical and virological characteristics of patients chronically infected with HBV or HCV.
| Parameters | HBV (n = 119) | HCV (n = 108) |
|---|---|---|
| Follow-up (years)*,† | 7.2 ± 3.1 | 7.5 ± 3.2 |
| (range) | (2.4–19.2) | (2.2–18.3) |
| Genotype 1‡ | - | 53 |
| Genotype 2‡ | - | 2 |
| Genotype 3‡ | - | 16 |
| Cirrhosis | 27 | 26 |
| AST (U/I)*,§ | 29.8 ± 18.4 | 56.2 ± 23.6 |
| ALT (U/L)*,§ | 31.4 ± 39.9 | 60.7 ± 44.4 |
| Antiviral therapy | 21 | 22 |
AST: aspartate aminotransferase. ALT: alanine aminotransferase. U/L: unities/liter.
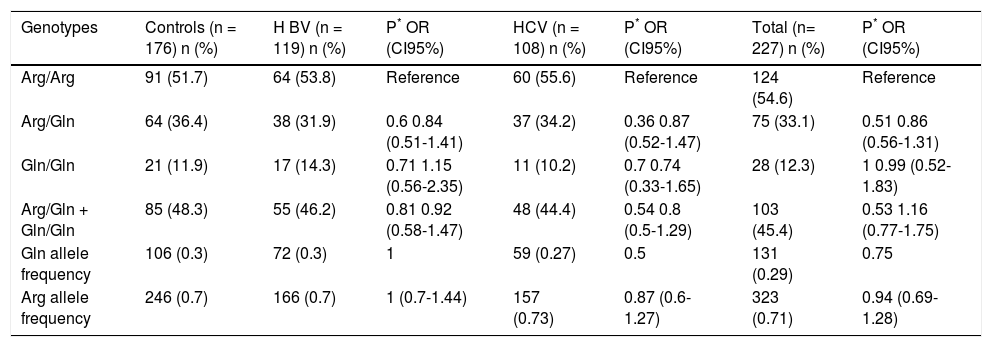
There was no difference in the genotypic frequencies (Arg/Arg, Arg/Gln or Gln/Gln) or allelic frequencies (Arg and Gln) between patients with viral hepatitis (either as a whole or in the HBV and HCV carriers separately) and the control group (Table 3); however, it was not possible to infer a role for this polymorphism in susceptibility because the control group was not age-matched with the patient group. The control group was in Hardy-Weinberg equilibrium, while the group of hepatitis viral patients was not.
Frequency of XRCC1 rs25487 genotypes and alleles in chronic hepatitis Β or C patients and controls subjects.
| Genotypes | Controls (n = 176) n (%) | Η BV (n = 119) n (%) | P* OR (CI95%) | HCV (n = 108) n (%) | P* OR (CI95%) | Total (n= 227) n (%) | P* OR (CI95%) |
|---|---|---|---|---|---|---|---|
| Arg/Arg | 91 (51.7) | 64 (53.8) | Reference | 60 (55.6) | Reference | 124 (54.6) | Reference |
| Arg/Gln | 64 (36.4) | 38 (31.9) | 0.6 0.84 (0.51-1.41) | 37 (34.2) | 0.36 0.87 (0.52-1.47) | 75 (33.1) | 0.51 0.86 (0.56-1.31) |
| Gln/Gln | 21 (11.9) | 17 (14.3) | 0.71 1.15 (0.56-2.35) | 11 (10.2) | 0.7 0.74 (0.33-1.65) | 28 (12.3) | 1 0.99 (0.52-1.83) |
| Arg/Gln + Gln/Gln | 85 (48.3) | 55 (46.2) | 0.81 0.92 (0.58-1.47) | 48 (44.4) | 0.54 0.8 (0.5-1.29) | 103 (45.4) | 0.53 1.16 (0.77-1.75) |
| Gln allele frequency | 106 (0.3) | 72 (0.3) | 1 | 59 (0.27) | 0.5 | 131 (0.29) | 0.75 |
| Arg allele frequency | 246 (0.7) | 166 (0.7) | 1 (0.7-1.44) | 157 (0.73) | 0.87 (0.6-1.27) | 323 (0.71) | 0.94 (0.69-1.28) |
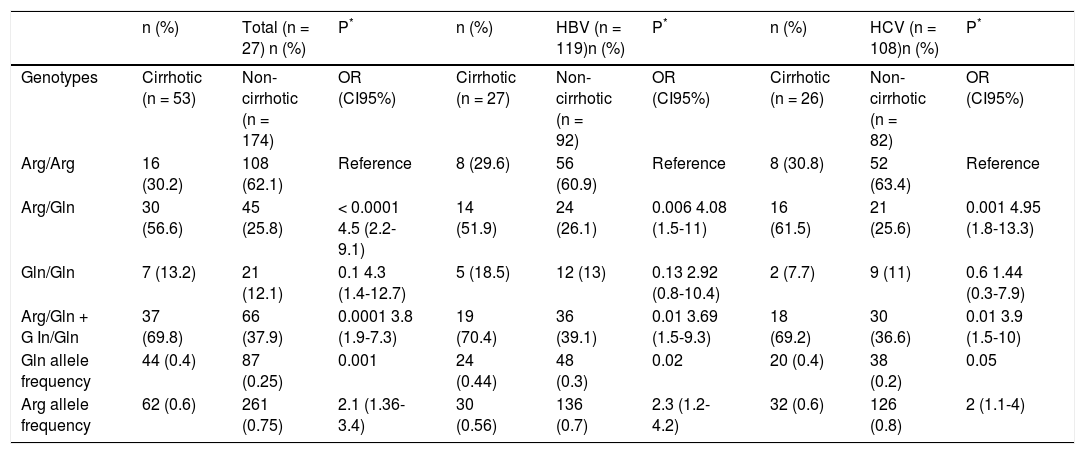
A significantly higher prevalence (p < 0.0001) of the Arg/Gln genotype was observed in the cirrhotic patients (56.6%) compared with the non-cirrhotic patients (25.8%). The association between this genotype and the presence of cirrhosis in patients remained statistically significant, independent of sex, age, alcohol intake and tabagism (adjusted OR = 3.5, CI = 1.7−7.4, p = 0.001). There was a significantly higher frequency of individuals with at least one Gln allele (Arg/Gln + Gln/Gln) in the cirrhotic group (69.8%) compared to the non-cirrhotic group (37.9%) (OR = 3.8, CI = 1.9−7.3, p = 0.0001). Again, this association was independent of the other analyzed factors (adjusted OR = 3.1, CI = 1.5–6.3, p = 0.002). The frequency of the Gln allele was significantly higher in the cirrhotic patients (0.4) than in the non-cirrhotic patients (0.25) (OR = 2.1, CI = 1.3–3.4, p = 0.001) (Table 4).
Frequency of XRCC1 rs25487 genotypes and alleles in chronic hepatitis Β or C patients with and without cirrhosis.
| n (%) | Total (n = 27) n (%) | P* | n (%) | HBV (n = 119)n (%) | P* | n (%) | HCV (n = 108)n (%) | P* | |
|---|---|---|---|---|---|---|---|---|---|
| Genotypes | Cirrhotic (n = 53) | Non-cirrhotic (n = 174) | OR (CI95%) | Cirrhotic (n = 27) | Non-cirrhotic (n = 92) | OR (CI95%) | Cirrhotic (n = 26) | Non-cirrhotic (n = 82) | OR (CI95%) |
| Arg/Arg | 16 (30.2) | 108 (62.1) | Reference | 8 (29.6) | 56 (60.9) | Reference | 8 (30.8) | 52 (63.4) | Reference |
| Arg/Gln | 30 (56.6) | 45 (25.8) | < 0.0001 4.5 (2.2-9.1) | 14 (51.9) | 24 (26.1) | 0.006 4.08 (1.5-11) | 16 (61.5) | 21 (25.6) | 0.001 4.95 (1.8-13.3) |
| Gln/Gln | 7 (13.2) | 21 (12.1) | 0.1 4.3 (1.4-12.7) | 5 (18.5) | 12 (13) | 0.13 2.92 (0.8-10.4) | 2 (7.7) | 9 (11) | 0.6 1.44 (0.3-7.9) |
| Arg/Gln + G In/Gln | 37 (69.8) | 66 (37.9) | 0.0001 3.8 (1.9-7.3) | 19 (70.4) | 36 (39.1) | 0.01 3.69 (1.5-9.3) | 18 (69.2) | 30 (36.6) | 0.01 3.9 (1.5-10) |
| Gln allele frequency | 44 (0.4) | 87 (0.25) | 0.001 | 24 (0.44) | 48 (0.3) | 0.02 | 20 (0.4) | 38 (0.2) | 0.05 |
| Arg allele frequency | 62 (0.6) | 261 (0.75) | 2.1 (1.36-3.4) | 30 (0.56) | 136 (0.7) | 2.3 (1.2-4.2) | 32 (0.6) | 126 (0.8) | 2 (1.1-4) |
When the patients infected with either HBV or HCV were considered separately, similar results were obtained for the HCV patients. In this group, both the heterozygote genotype and the presence of at least one Gln allele were significantly associated with cirrhosis, independent of sex, age, alcohol intake and tabagism (adjusted OR=3.82, CI=1.4-10.7, p=0.01 and OR=3.1, CI=1.1-8.4, p=0.03). However, in the HBV-infected patients, the association initially observed for heterozygotes and Arg/Gln + Gln/Gln did not show independence from the other variables considered (p = 0.06, adjusted OR = 3, CI = 0.9-9.6 to Arg/Gln and p = 0.06, adjusted OR = 3, CI = 0.9–9.6 to Arg/Gln + Gln/Gln) (Table 3). In addition to the XRCC1 rs25487 polymorphism, only age was associated with cirrhosis in all of the groups analyzed.
As the levels of AST and ALT are biochemical markers of liver damage, we compared these parameters between the patients who are homozygotes Arg/Arg and those who are Arg/Gln + Gln/Gln. In HBV-infected patients, the levels of AST or ALT were similar both in the Arg/Arg homozygotes (29.78 ± 18.4 and 33.57 ± 27.61) and in the Arg/Gln + Gln/Gln genotypes (31.37 ± 23.55 and 32.83) (p = 0.48 and p = 0.13, respectively). The HCV-infected patients and the Arg/Gln + Gln/Gln genotype presented higher levels of AST (64.78 ± 46.36) and ALT (65.44 ± 41.50) in comparison to the Arg/Arg homozygotes (49.25 ± 32.56 and 56.76 ± 46.70, respectively); however, these differences were not statistically significant (p = 0.07 and p = 0.09).
DiscussionThe progression of hepatic disease in individuals with chronic viral hepatitis is associated with an increase in oxidative stress and an accumulation of DNA damage.14–16 Additionally, genetic polymorphisms in genes encoding proteins involved in DNA repair may influence an individual’s susceptibility to cancer and chronic inflammatory disease.3,10,19,20,24,25 However, few studies have investigated the influence of polymorphisms in DNA repair genes in patients with viral hepatitis. In this pilot study, the possible influence of the XRCC1 rs25487 polymorphism on the susceptibility to cirrhosis in viral hepatitis patients was investigated. Despite the small sample size, the results obtained are quite interesting and suggest that the XRCC1 rs25487 polymorphism may play a role in the development of more aggressive diseases in these patients.
The control group was in Hardy-Weinberg equilibrium, but this equilibrium was not observed for the hepatitis patients. Although the departure from the Hardy-Weinberg equilibrium in the non-affected group (control) is commonly considered a genotyping error in case-control studies, it may be expected in the affected group (patients) if the locus confers susceptibility to common diseases.26,27 There were no differences in the genotype and allelic frequencies between patients with viral hepatitis and the control group. The frequencies of the Gln allele in the studied groups (which ranged from 0.27 to 0.3) were similar to those described in the Southeastern and Northern Brazilian regions.19,21,28
A significant association was observed between patients with cirrhosis and both the heterozygote genotype, Arg/Gln + Gln/Gln, and the Gln allele, and this association was independent of age and the other variables considered (Table 4). An association between this polymorphism and cirrhosis in viral hepatitis patients has not been previously described, to the best of our knowledge. However, an increase in the relative risk of alcoholic liver cirrhosis was described in association with the heterozygous and Gln/Gln homozygous genotypes in older Southeastern Brazilians,21 indicating that this polymorphism may contribute to this condition.
Establishing a relationship between the efficiency of DNA repair and the incidence of cirrhosis is difficult as there is no direct evidence that DNA damage contributes to cirrhosis. Some indirect evidence, however, indicates that DNA damage may contribute to the progression of hepatic lesions in HCV. Oxidative DNA damage in leukocytes and hepatocytes is elevated in HCV patients and is correlated with the presence and extension of liver damage.15,16 Furthermore, there is evidence that apoptosis is related to the presence of hepatic lesions in HCV infection, with apoptosis being more frequent in patients with high stage fibrosis.29 The XRCC1 rs25487 polymorphism has been associated with less efficient DNA repair, which in turn may result in apoptosis.8,17 The activity of transaminases in the serum is a marker of liver damage and often correlates with the presence of apoptotic markers in the serum.30 In this study, the levels of AST and ALT were not significantly influenced by the presence of the Gln allele in HBV or HCV-infected patients; however, it is interesting that the HCV-patients with at least one Gln allele presented with higher levels of these transaminases than the Arg/Arg homozygotes did. Whether the XRCC1 rs25487 polymorphism may indirectly contribute to increased apoptosis and, consequently, to the liver damage in viral hepatitis patients is a matter that will require further study.
In conclusion, the results presented here suggest that the XRCC1 rs25487 polymorphism may influence the development of cirrhosis in patients with viral hepatitis. The sample size is limiting in this study. However, as preliminary data, the results presented here are a promising first step in establishing a relationship between DNA repair inefficiency and the development of cirrhosis.
Abbreviations- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
HBV: hepatitis B virus.
- •
ROS: reactive oxygen species.
- •
XRCC1: X-ray repair cross complementing protein 1.
This research was supported by Fundaçᾶo de Amparo à Pesquisa do Estado do Mato Grosso- FAPEMAT (grant number 737379/2008).
AcknowledgementsWe would like to thank Gevanil Arruda, Nilson Santana Botelho and Maria Celina da Penha for technical assistance and the Brazilian Research Council, Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq and Coordenaçᾶo de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES) for awarding a fellowship to the authors (Leite, STAP, Guimaráes NA, Oliveira, JCS and Bassi-Branco, CL).