Elevated serum ferritin, or hyperferritinemia, is a common finding on routine bloodwork and often prompts referral for further evaluation. In the following review, we outline the various causes of hyperferritinemia and point out that, in the majority of cases, this does not represent true iron overload. Despite much research interest in this area, the precise mechanism of hyperferritinemia and its impact on disease severity in various clinical conditions continues to be debated. While some research suggests that iron reduction in cases of hyperferritinemia is of benefit, the decision to treat such patients should be individualized, and may be influenced by the presence of other features of iron overload.
Serum ferritin is the most frequently requested measure of body iron status and one of the most frequently requested laboratory tests in both primary care and referral settings.1 Ferritin is the cellular storage protein for iron and is able to store up to 4,500 atoms of iron with much of this iron accessible for metabolic needs. The normal adult total body iron content is approximately 3-4 g. On average, 2.5 g is contained within hemoglobin in circulating red blood cells and developing erythroblasts, 400 mg in iron containing proteins, 3-7 mg in the form of transferrin-bound iron and the remainder stored as ferritin or hemosiderin.
The human body has no means of excreting excess iron. Only a very small amount, average 1 mg, leaves the body each day via loss in sweat, shed skin cells and some gastrointestinal loss. The vast majority (20-25 mg) is efficiently recycled from the breakdown of senescent red blood cells within the reticuloendothelial system. The average Western male consumes 1-2 mg of heme iron and 10-15 mg of non-heme iron each day. Roughly 30% of heme iron is absorbed and 10% of non-heme iron, totaling 1-2 mg/day maintain iron balance.
Ferritin is a reliable surrogate marker of body iron stores with low values providing absolute evidence of reduced reticuloendothelial iron stores.2 Elevated ferritin levels however, are far less specific for systemic iron overload. Ferritin is an acute phase reactant, defined as a protein whose serum concentration increases or decreases by at least 25 percent during inflammatory states.3
The magnitude of the problem of hyperferritine-mia was evidenced by the results of the HEIRS stu-dy.4 This population based screening study sought to determine the incidence of hereditary hemochro-matosis (HH) in a large multi-ethnic, multi-racial primary care setting within North America. Of the 101,168 participants screened for iron overload and HFE mutations, 5.9% of Caucasian subjects and 19% of Asian subjects were found to have hyperfe-rritinemia. Whereas 0.44%, (approximately 1 in 200) of Caucasians were found to be homozygous for the C282Y mutation only 0.00004% (1 in 25,000) Asians were homozygous. This important study demonstrated that hyperferritinemia is very common and that in the majority of individuals it is due to conditions other than HH.
What is a Normal Ferritin?When approaching the diagnosis of hyperferriti-nemia one must first determine what a “normal” value is. Reference ranges vary across laboratories due to differences in analytical techniques and reference populations. Age and sex are also important determinants with lower cutoffs acceptable for women due to increased iron loss secondary to menses, pregnancy and lactation. The HEIRS study defined that a serum ferritin concentration > 300 μg/L in men and > 200 μg/L in women was elevated. However, if the reference range had been determined from the HEIRS study as mean ± two standard deviations, the upper limit of the reference range would have been significantly > 300 μg/L. Generally levels between 30-300 μg/L for men and 15-200 μg/L for women are considered normal, which reflects the significant variation in serum ferritin present in the population.
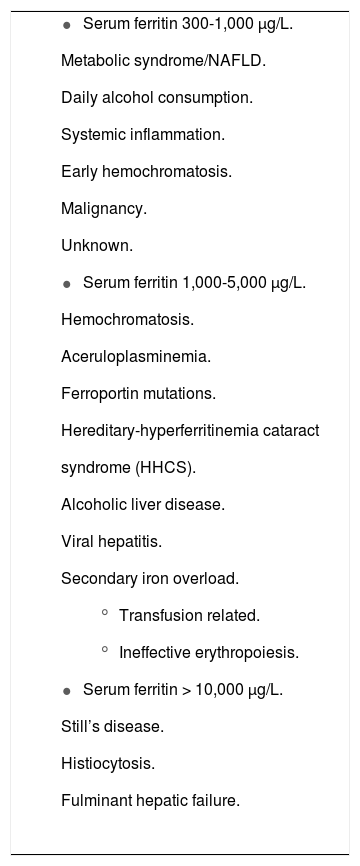
Not All Hyperferritinemia Represents Iron OverloadHyperferritinemia can be caused by a variety of systemic conditions including infection, neoplasm and chronic or acute inflammation (Table 1). In the setting of C282Y-linked hemochromatosis, ferritin is a sensitive marker of iron overload and is used to monitor response to treatment. However, ferritin elevations may also be commonly seen in a number of other liver diseases including alcoholic and nonalcoholic steatohepatitis (NASH), viral hepatitis and almost any cause of acute liver injury will be accompanied by ferritin elevation.
Causes and spectrum of hyperferritinemia.
|
Elevated serum ferritin levels are increasingly being recognized in patients with features of the metabolic syndrome and insulin resistance. This is particularly relevant in the setting of NASH, which is the hepatic manifestation of the metabolic syndrome. Whether hyperferritinemia itself is associated with more severe liver disease in NASH pa-tients remains controversial. As well, whether targeting high serum ferritin for treatment via phlebotomy improves liver disease is as yet somewhat uncertain.
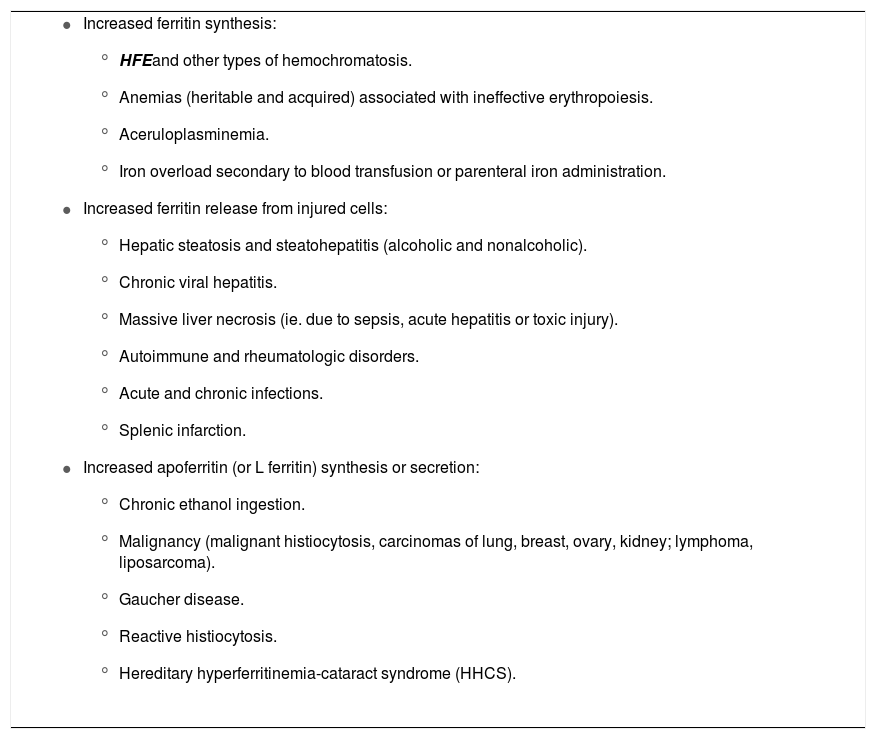
One of the greatest challenges facing physicians dealing with patients with hyperferritinemia is determining whether the elevated ferritin truly represents iron overload. As stated previously, hyperferritinemia can be seen in a wide variety of clinical conditions, many of which do not cause iron overload. The cause of elevated serum ferritin is usually the result of increased ferritin synthesis from acquired or genetic disorders, or increased release of ferritin from damaged cells (Table 2).
Mechanisms of hyperferritinemia in various disorders.
|
In order to determine the cause of ferritin elevation, the clinical setting can be very helpful. Patients with a history of chronic alcohol ingestion or features of the metabolic syndrome (obesity, insulin resistance, dyslipidemia and hypertension) often present with hyperferritinemia. Similarly, patients with chronic inflammatory conditions, renal insufficiency, malignancy and marrow failure may present in a similar manner. In each of these groups ferritin elevation is typically moderate (< 1,000 μg/L) and transferrin saturation (TS) is normal. Systemic iron overload is typically not present and the approach to treating these patients relies on identifying and treating the underlying cause appropriately. A recent review of causes of hyperferritinemia in patients referred to a tertiary care center for evaluation of elevated serum ferritin revealed that causes other than HFE-linked hemochromatosis were found in more than one-half of cases5 (Table 3).
Clinical assessment of the cause of the elevated serum ferritin. Iron overload more likely-C282Y homozygote, Transferrin saturation elevated, Ferritin rising over time, Family history of iron overload.
| Iron overload less likely-Modest ferritin elevation (< 1,000 μg/L), normal transferrin saturation, underlying liver disease or inflammation, daily alcohol consumption, obesity. |
| Diagnostic assessment for iron overload. HFE genotyping, MRI scan, liver biopsy, trial of phlebotomy. |
| Phlebotomy treatment for an elevated serum ferritin |
| Pro - |
| • Normalization of serum ferritin may reduce liver iron and possible liver injury. |
| • Improvements in insulin resistance and prevention of vascular disease. |
| • Patients are reassured as numbers normalize. |
| • Blood donor pool may increase. |
| • The most compelling indication is in the C282Y homozygote where iron overload and liver injury secondary to iron overload are clearly established. |
| Con - |
| • A large number of patients without iron overload will have phlebotomy. |
| • Iron deficiency anemia can occur in this setting. |
| • Treatment reinforces a diagnosis of iron overload which may not be present. |
| • Phlebotomy treatment requires resources and health care systems may have higher priorities. |
In patients whom have elevated serum liver enzymes in addition to hyperferritinemia, the diagnostic approach should include genetic testing for HH. Whereas the majority of patients with HH will have concomitant elevation of TS this is not always the case. It has been shown that serum iron indices demonstrate diurnal variation and that TS exhibits significant within-person biological variability which limits its usefulness as an initial screening test for HH.6,7 Transferrin saturation is a calculated value which represents the quotient of the serum iron level divided by one of the following: total iron binding capacity; unsaturated iron-binding capacity + serum iron con-centration; or serum transferrin concentration multiplied by a constant. The fact that determination of TS is a two-step process, relying on the measurement of a number of other variables may explain some of its variability. Liver disease can occasionally cause low serum transferrin concentrations which may lead to increased TS without iron overload.
Attempts to find a Better Ferritin TestThere are biochemical variations in the types of ferritin circulating in the human plasma. Tissue fe-rritins differ from serum ferritin in that they are typically glycosylated.8 Serum ferritin is a secreted protein, whereas tissue ferritins leak from damaged cells. Ferritin can differ in H and L subunits and in the amount of iron carried per molecule.9 These differences can be determined by isoelectric focusing and other analytical methods but they have not been widely used to differentiate iron overload from a ferritin related to inflammation.10 The use of surrogate markers; such as CRP for inflammation or GGT for alcohol have been used and are generally only helpful if elevated.
AIDS in Determining Whether Hyperferritinemia Means Iron OverloadTransferrin saturation elevation is central to the pathogenesis of iron loading in HH. Elevated TS results in part from increased iron absorption, and increased iron release from macrophages into plasma. Our centre represents a diverse, tertiary care population. We recently evaluated the correlation between serum TS and liver iron concentration (LIC) in a large sample of patients with and without iron overload. This demonstrated that although elevated TS was often seen in the setting of iron overload, there are a significant number of patients, 44/191 (23%) in this cohort, with an elevated ferritin and normal TS that have an elevated LIC.11 It was concluded that a normal TS should not be used to exclude the diagnosis of hemochromatosis, but that both TS and serum ferritin should be used together to guide further investigations such as HFE genetic testing and/or liver biopsy.
HFE genetic testing is often part of the evaluation of hyperferritinemia. Some HFE C282Y homozygotes, and less commonly HFE H63D homozygotes and compound heterozygotes (HFE C282Y/H63D) develop iron overload.4 Additional mutations in other iron related genes such as hemojuvelin, hepcidin, ferropor-tin and transferrin receptor 2 are recognized, but as yet, there is no widely available commercial test for these mutations.12
Liver biopsy has long been considered the gold standard in evaluating hepatic iron overload and also allows for assessment of concomitant liver disease. However, sampling error is of concern, as a single biopsy represents only 1/50,000th of the li-ver.13 Studies of paired biopsies have shown that significant variability can occur between different regions of the liver.14 An indirect mode of measuring liver iron content is MRI which relies on the pro-magnetic properties of storage iron (ferritin and he-mosiderin). MRI has shown promise in the accurate, non-invasive measurement of liver iron and as a means of assessing response to therapy for iron overload.15,16
Does Hyperferritinemia Have an Adverse Effect on Health?In recent years there has been a great deal of interest regarding the relationship between markers of inflammation and health outcomes. It is now well recognized that activation of inflammatory mediators plays a significant role in the pathogenesis of many disease processes that had previously been thought to be unrelated to inflammation. These include type 2 diabetes (DM2), obesity and other conditions comprising the metabolic syndrome (MS). As well there is increasing evidence supporting the role of inflammation in vascular disease.
As stated previously, hyperferritinemia is considered an acute phase reactant and as such is elevated in states of systemic inflammation. A number of studies have evaluated the association of hyperferri-tinemia with disease risk and outcome. Two recent large, population based studies found that high serum ferritin levels are associated with an increased risk of MS and DM2.17,18 Importantly, this increased risk was present among non-obese adults, a group whom would not normally be considered at significant risk for these conditions.
There is also some evidence to support a link between excess iron and malignancy, specifically hepa-tocellular carcinoma (HCC). The increased risk of HCC is well recognized in patients with HH.19 Even in the absence of HH, individuals with excess liver iron have a higher risk of HCC.20
The relationship between hyperferritinemia and vascular disease has also been supported by recent studies. A cohort of otherwise healthy young men was evaluated to determine whether elevated serum ferritin was associated with decreased levels of cardiovascular fitness (CVF).21 Interestingly, serum fe-rritin levels > 150 μg/L, which is lower than what is normally considered to be elevated, were associated with poorer CVF. Additional studies have examined the relationship between ferritin levels and peripheral artery disease (PAD), concluding that hyperferritinemia is associated with the presence of PAD, and in one study was associated with an increased risk of mortality from PAD.22,23
Likely the area of greatest interest regarding a potential relationship between hyperferritinemia and disease is in the setting of nonalcoholic fatty liver disease (NAFLD). Support for this comes from the results of large, population based studies. In the Nurse’s Health Study of over 30,000 healthy women, it was concluded that and elevated serum ferri-tin was associated with an increased risk of type 2 diabetes.24 Similar results were noted in cohort of over 6,000 patients participating in the Third National Health and Nutrition Examination Survey (NHANES III) where the metabolic syndrome was significantly more common in those with and elevated serum ferritin.25 A recent study demonstrated that elevated serum ferritin was associated with worsened histologic activity of NAFLD and was an independent predictor of advanced hepatic fibrosis.26 The term “dysmetabolic iron overload syndrome” has even been coined to describe this subgroup of NAFLD patients.
Studies have been published regarding the potential pathophysiologic role of iron in NAFLD.27,28 One widely supported view is that iron accumulation leads to increased oxidative stress and lipid peroxi-dation, both of which are central to the pathophy-siology of NAFLD. Iron is capable of catalysing the formation of reactive oxygen (ROS) species and a number of studies have demonstrated that hepatic iron loading leads to oxidative damage.29,30 Hepatic stellate cells can be activated by ROS and thus, by this mechanism, iron may induce fibrogenesis.31 However, most patients with an elevated ferritin with NASH have a normal liver iron concentration which does not support the theory of iron overload leading to oxidative stress.
Are We Treating Disease, Preventing Disease or Treating a Number?These associations beg the question that if hyper-ferritinemia is associated with a particular disease state, then does treating hyperferritinemia improve outcomes in such conditions? Unfortunately, the answer to this question is not entirely clear.
A large multicenter, randomized controlled trial was conducted evaluating iron reduction (phlebotomy) as a treatment for patients with symptomatic but stable PAD.32 A total of 1,277 patients were randomized to either phlebotomy or control. No significant differences were noted between the two groups for either the primary outcome of all-cause mortality or secondary outcome of death plus nonfatal myo-cardial infarction and stroke. However, a more recent study by this same group has suggested that a lower iron burden may improve survival and prevent or delay nonfatal myocardial infarction and stroke.33
The therapeutic role of iron depletion in patients with liver disease may be somewhat more promising. Stainable iron in hepatocytes and portal tracts has been shown to be a predictor of disease progression in hepatitis C, including HCC and death.34 This may be a sequelae of liver disease severity rather than a cause. Iron depletion has also been suggested to lower the risk of HCC in these patients.35
Iron excess in the setting of NAFLD and the MS is an area of significant research interest. Whereas HCC is a rare complication of cirrhosis due to NAFLD, like hepatitis C, there is some evidence suggesting that hepatic iron deposition increases the risk of this tumor.36,37 Removal of iron by phlebotomy has been shown to decrease markers of oxida-tive stress and levels of TGF-β1, a known stimulant of stellate cell proliferation and fibrogenesis.38 Some studies of iron reduction therapy have shown improvement in transaminases and metabolic indices such as insulin resistance. However, its impact on hepatic steatohepatitis is largely unknown.39–41 A prospective study of phlebotomy evaluating pre and post liver histology is currently underway at our center in order to address this important question (NCT00641524).
Is there a rationale for phlebotomy if it is not likely to be iron overload?Unfortunately there is still not an absolute answer to this question. The short answer is likely to be no, but as discussed in previous sections of this review, the area is still not fully understood.
Whereas the role of phlebotomy remains controversial in the absence of true iron overload, phlebotomy is likely a reasonable course of action in those patients with demonstrable iron excess. It is clear that iron excess cannot be accurately diagnosed based solely on elevated serum ferritin. It is our recommendation that the presence of tissue iron deposition be confirmed by at least one of the following modalities; iron staining on liver biopsy, measurement of liver iron concentration (LIC) or alternatively, MRI which offers an accurate, nonin-vasive alternative to liver biopsy. In cases where iron excess is confirmed in the setting of high ferri-tin, research to date supports a beneficial effect of iron reduction therapy.
Support for this approach comes from preliminary results of our prospective trial of phlebotomy in NA-FLD patients. Whereas 64% had an elevated serum ferritin, only 8% had an elevated LIC (defined as > 36 μmol/g). The ratio of serum ferritin to liver iron is much higher in NAFLD than that seen in HH. This suggests that a component of the serum ferritin seen in NAFLD is a reaction to inflammation.
Empirical phlebotomy and areas of uncertaintyMany patients (and physicians) express alarm at the news of an elevated serum ferritin. The initial step in the management should be to point out to the patient that there are many causes of an elevated ferritin and iron overload is not the most likely cause in the general population where inflammation, obesity and alcohol would be more likely causes. It may also be helpful to point out that an elevated serum ferritin is extremely common and that ferritin elevations < 1,000 μg/L are considered modest. Many patients seem determined to die with normal lab test results and are enthusiastic about phlebotomy therapy. These patients should be encouraged to be voluntary blood donors but often are ineligible because of co-morbid diseases and medications. To initiate phlebotomy therapy in the large numbers of patients with an elevated ferritin is resource intensive and the feasibility will depend on the health care system. Many patients enjoy phlebotomy therapy and report an improvement in fatigue and there may be a psychosocial support component to this treatment. A number of areas of uncertainty remain in this clinical setting. If the ferritin elevation is considered to be inflammatory in nature, why does it decrease with phlebotomy? If the liver iron concentration is normal in most of these patients, how does phlebotomy decrease oxidative stress? Could there be other additional medical benefits of phlebotomy unrelated to iron or ferritin?
ConclusionsHyperferritinemia is very common and mild elevations (300-1,000 μg/L) are frequent in clinical practice. A careful clinical assessment, including a detailed history for alcohol consumption as well as metabolic risk factors (obesity, DM2, dyslipidemia and hypertension) will reveal the cause in many individuals. While the cause of hyperferritinemia is not fully understood in these conditions, evidence to date suggests that inflammation plays a role and that in many cases significant hepatic iron deposition is absent. Based on the preliminary results of our study of phlebotomy in NAFLD we suggest that the currently accepted reference ranges for serum ferritin in healthy adults may be inappropriate in this group and investigations, such as liver biopsy, for iron overload in NAFLD patients with ferritin < 1,000 μg/L is low yield.
Among patients with demonstrated hepatic iron deposition, the benefit of phlebotomy is well established in HH. Evidence suggests that this therapeutic approach may be beneficial in a number of other conditions including NAFLD. However, this has not yet been confirmed by prospective pre and post phlebotomy biopsy data. As well, the mechanism by which disease improvement may occur with phlebotomy is not fully understood. Additional studies are clearly needed to further define the role of ferritin in disease.
Abbreviations- •
HH: hereditary hemochromatosis.
- •
NASH: nonalcoholic steatohepatitis.
- •
TS: transferrin saturation.
- •
LIC: liver iron concentration.
- •
DM2: type 2 diabetes mellitus.
- •
MS: metabolic syndrome.
- •
HCC: hepatocellular carcinoma.
- •
CVF: cardiovascular fitness.
- •
PAD: peripheral artery disease.
- •
NAFLD: nonalcoholic fatty liver disease.
- •
ROS: reactive oxygen species.