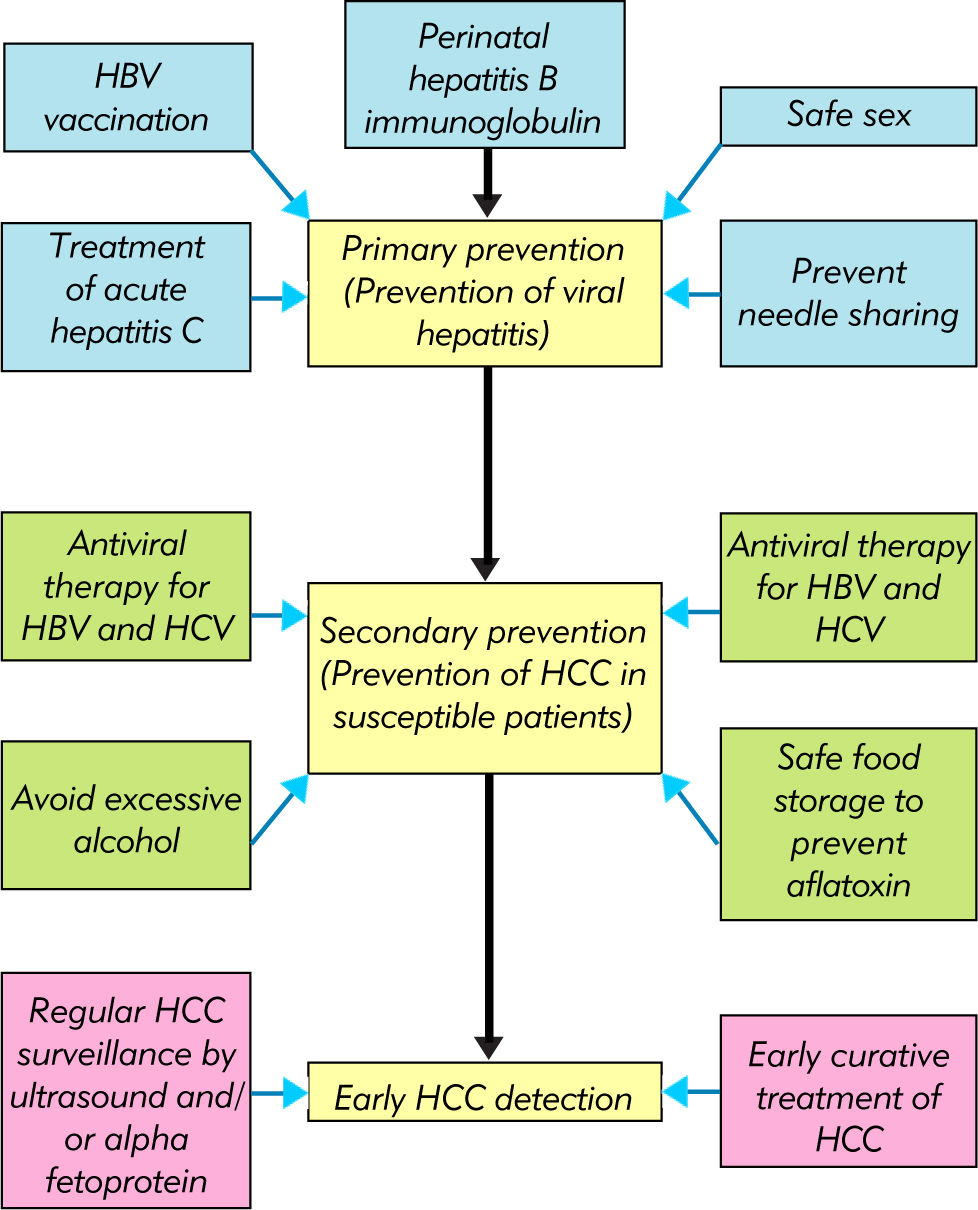
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer deaths in men. Due to differences in the prevalence of viral hepatitis, the incidence of HCC in low and middle income countries is much higher than that of high income countries. Strategies to limit the impact of HCC include primary prevention against new cases of viral hepatitis, secondary prevention of HCC in susceptible individuals, and early HCC detection. Universal hepatitis B vaccination has resulted in dramatic reduction in incident cases of chronic hepatitis B and HCC in children and adolescents, and the full effect is expected in the next 20 years. The key hurdle for universal vaccination is the cost and the accessibility in low and middle income countries. Randomized controlled trials and meta-analyses showed that successful treatment of chronic hepatitis B and C can reduce the risk of HCC and cirrhotic complications. HCC surveillance by regular ultrasound examination and alpha fetoprotein testing leads to early cancer detection and offers the opportunity for curative treatment. Since all these measures are costly and require manpower and infrastructure support, the implementation should rely on the liaison among healthcare providers and policymakers. The cost-effectiveness of various strategies should also be studied based on local situations.
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer deaths in men worldwide.1 Due to geographical differences in the underlying etiologies such as viral hepatitis and alcoholism, the global distribution of HCC is uneven. The incidence and mortality of HCC in low and middle income countries are much higher than those of high income countries. Since late stage HCC is almost uniformly lethal, the key to the control of the disease includes prevention and early cancer detection. This review focuses on the impact of vaccination programs and antiviral therapy in the prevention of HCC, as well as the efficacy of HCC surveillance programs. The practical and financial implications of such programs in developing countries are discussed.
Epidemiology OF HCCAccording to GLOBOCAN, there were approximately 748,300 new cases of primary liver cancers in 2008.1 695,900 people were estimated to die from primary liver cancers, indicating that most of the new cases would eventually die from the disease. HCC accounts for 85 to 90% of all primary liver cancers.2
Among 748,300 new cases of primary liver cancers in 2008, 626,700, or 84%, occurred in developing countries. The incidence is highest in Eastern Asia (age-standardized incidence rates 35.5/100,000 in men, 12.7/100,000 in women). Other high-rate areas include:
- •
Southeast Asia (21.4/100,000 in men, 9.0/100,000 in women),
- •
Middle Africa (18.9/100,000 in men, 9.6/100,000 in women),
- •
Western Africa (16.6/100,000 in men, 8.0/100,000 in women), and
- •
Southern Africa (13.9/100,000 in men, 5.1/ 100,000 in women).
In contrast, the incidence is less than 10.0/ 100,000 in men and 5.0/100,000 in women in most parts of Europe and North America. Corresponding figures are 6.3/100,000 in men and 4.4/100,000 in women in Caribbean countries, and 5.3/100,000 in men and 3.9/100,000 in women in South America. Male preponderance is consistently observed among different countries, with male-to-female ratio between 2:1 and 4:1.
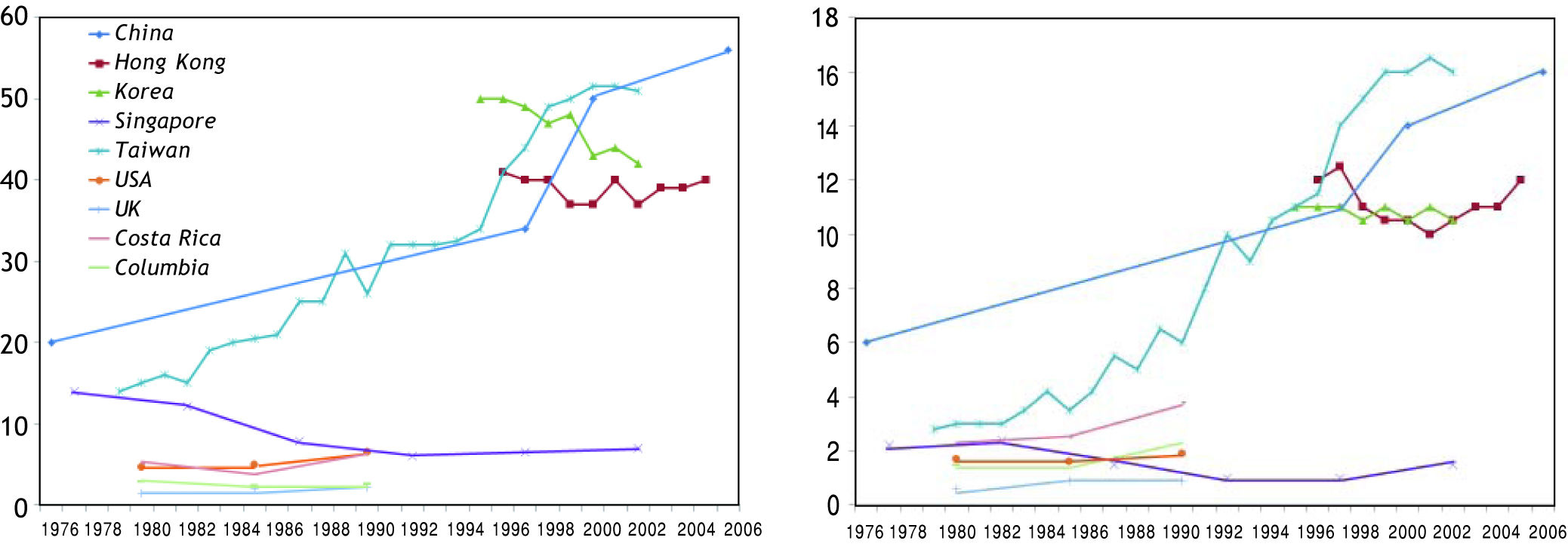
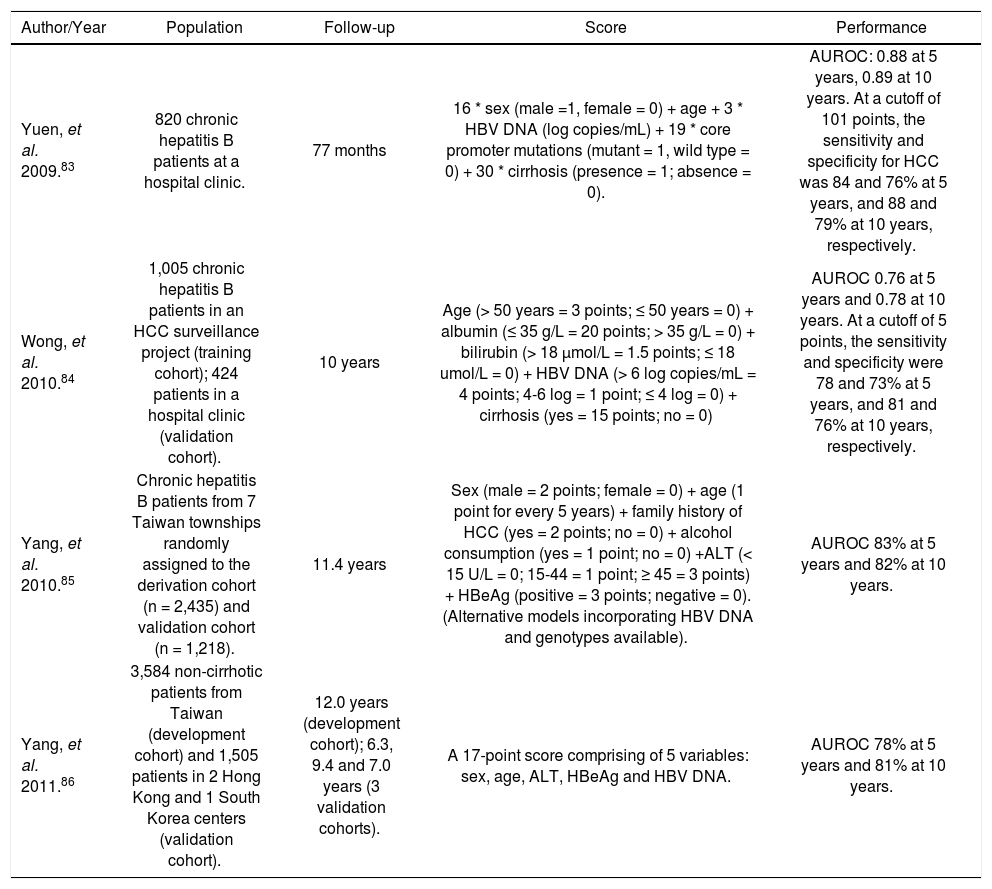
The trend of HCC also differs much across various countries (Figure 1). In some parts of America, the incidence has increased by more than 2-fold from 1970s to late 1990s.3,4 In Asia, the incidence is static or decreasing in some areas like Korea, Singapore and Hong Kong.5 The phenomenon is not completely understood. In some Western countries, the increase can be partly explained by immigration from countries with high prevalence of liver diseases. An alternative explanation is that countries with rising incidence in HCC tend to have higher prevalence of hepatitis C virus (HCV) infection. Besides, the increasing incidence of obesity and diabetes may have contributed to HCC risk in some countries.
Hepatitis B Virus and HCCIt is estimated that over 350 million people are chronically infected with hepatitis B virus (HBV) worldwide.6 Up to 25% of these patients would eventually die from liver disease. Globally, HBV accounts for 30 and 53% of all cases of cirrhosis and HCC, respectively.7 Among different liver diseases, chronic hepatitis B is unique in that HCC may develop in a liver with little or no fibrosis. This is because HBV is directly carcinogenic, partly explained by the integration of HBV DNA into the human chromosomes and the action of HBx regulatory pro-tein.8,9
VaccinationThe first HBV vaccine was developed from human serum and licensed by the Food and Drug Administration of the United States in 1981.10 This was subsequently replaced by the development of recombi-nant yeast (Saccharomyces cerevisiae) vaccine that expresses HBsAg in 1984.11 The vaccination is given in 3 doses as intramuscular injections. The first and second doses are given 1 to 2 months apart. The third booster dose is given 6 to 12 months later.
The response to vaccine declines with age. As the prevalence of HBV infection in most middle and low income countries is high, childhood immunization, preferably since birth, should be recommended. After completion of 3 doses of vaccine, 90% of healthy adults and over 95% of children would develop protective antibodies. Infants of mothers with chronic hepatitis B derive further protection with the addition of hepatitis B immune globulin. Positive HBsAg is found in only 6% of infants who receive both hepatitis B immune globulin and vaccine, compared to 29% of those receiving immune globulin alone, 25% of those receiving vaccination alone, and 88% of those without prophylaxis.12
Although the antibody level against HBsAg tends to wane with time, this does not appear to be important clinically. In a 12-year follow-up study of Senegalese infants, HBsAg carrier rate remained low among vaccinated children.13 Further boosters at school did not affect the protection rate. The strongest evidence comes from epidemiological studies that show no increase in HBsAg carrier rate in adolescents who have received vaccination years ago.14,15 This indicates that new HBV infection is exceedingly rare in vaccinated persons.
Alongside with a reduction in HBsAg carrier state, the incidence of HCC in vaccinated persons has also declined. Based on experience in Taiwan, the annual incidence of HCC in children 6 to 14 years of age declined from 0.70 per 100,000 children between 1981 and 1986 to 0.57 between 1986 and 1990, and to 0.36 between 1990 and 1994.16 A follow-up study confirmed that the protection against HCC had extended to adolescents up to the age of 19 years.17 Because of this cohort effect, the true impact on the population incidence of HCC would only be clear at least 20 years after the introduction of mass vaccination. When the vaccination cohort reaches adulthood, the population incidence of HBV infection and HCC is expected to decrease dramatically.
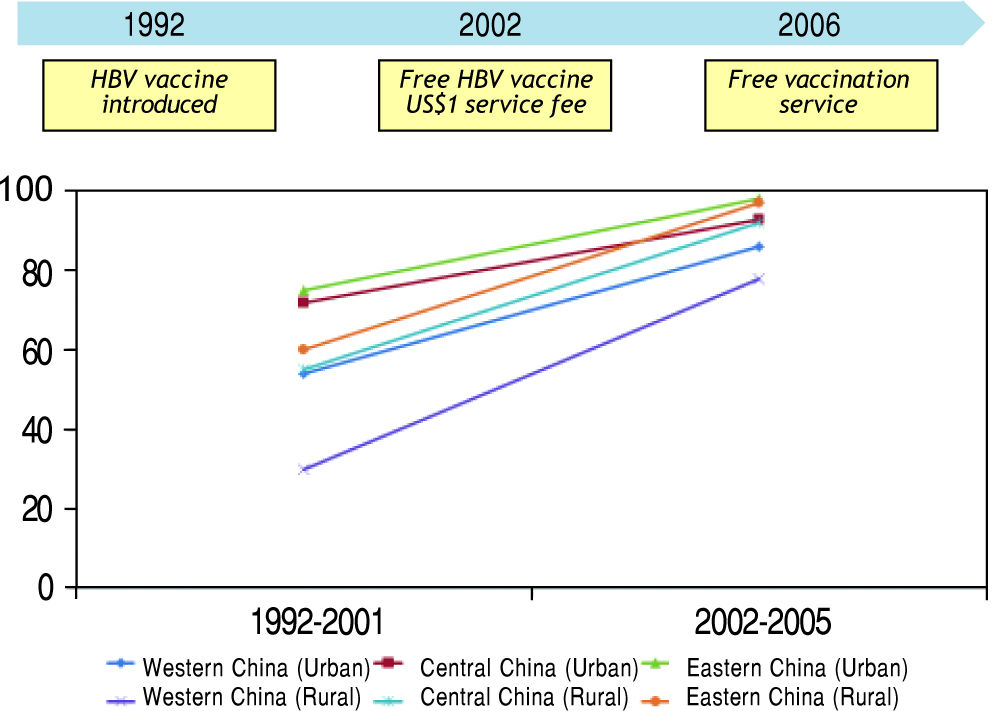
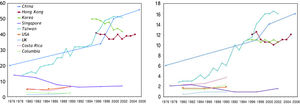
According to a recent Asia-Pacific working party consensus statement, universal vaccination to HBV is the most important preventive strategy against HBV-related HCC.18 However, this program is only effective if infants are really receiving the vaccine (Figure 2). The experience from mainland China illustrates how public health policy influences the effectiveness of the vaccination program. When HBV immunization was introduced in 1992, the families had to pay for the vaccine. The vaccine coverage at that time was only 30%, and particularly worse in poor and rural areas.19 In 2002, the government decided to support free HBV vaccine, but the families still need to pay around US$1 as service charge. It was not until June 2005, when the government waived all vaccination-associated charges, the vaccination coverage in children increased dramatically to 90%. As a result, the population prevalence of HBsAg has declined from 9.8% in 1992 to 7.2% in 2006. In children below the age of 5, the prevalence has decreased from 9.7% in 1992 to 1.0% in 2006.20 Another challenge is the accessibility of vaccine in rural area. For example, the vaccine coverage in the poorer Western part of China is always lower than the Eastern part, even when the vaccination is completely free of charge.19 Another example is Vietnam, where only 70% of the provinces have implemented vaccination into the expanded immunization program in 2004.21
Antiviral therapyHigh serum alanine aminotransferase and HBV DNA levels, persistently positive hepatitis B e antigen (HBeAg) and liver cirrhosis are associated with disease progression and HCC development.22–24 Antiviral therapy effectively controls these risk factors and is expected to improve clinical outcomes. The best evidence on the efficacy of oral antiviral drug came from an Asian multicenter trial.25 Six hundred and fifty-one patients with histology-proven advanced fibrosis or early cirrhosis were randomized to receive lamivudine (n = 436) or placebo (n = 215). At a median follow-up of 32 months, disease progression, defined by hepatic decompensation, HCC, spontaneous bacterial peritonitis, bleeding varices, or liver-related death, occurred in 7.8% in the lamivudine group and 17.7% in the placebo group (p = 0.001). HCC occurred in 3.9% in the lamivudine group and 7.4% in the placebo group (p = 0.047). Unfortunately, the study was originally planned for 5 years but was prematurely terminated at around 3 years because of significant difference in the primary end point. This resulted in inadequate power to assess individual outcomes. For example, although the difference in HCC reached borderline statistical significance, the result was no longer significant if cases of HCC occurring during the first year of study were excluded. Controversies also exist among patients who have advanced liver cirrhosis. In two randomized, controlled trials (entecavir vs. adefovir and telbivudine vs. lamivudine, respectively), more potent viral suppression among patients suffering from advanced liver cirrhosis (Child’s grade B or C) was not translated to a reduction of HCC or improvement in survival.26,27
A meta-analysis of 12 studies (n = 2,742) showed that interferon reduced the risk of HCC by 34% compared to no treatment or placebo.28 Similarly, analysis of 5 studies (n = 2289) showed that lamivu-dine reduced the risk of HCC by 78%. Another meta-analysis of 11 studies showed that interferon was effective in reducing the risk of other cirrhotic complications as well.29 Overall, patients with histologi-cal and biochemical improvements with antiviral therapy are less likely to develop cirrhotic complications and HCC.30
The costs and reimbursement policy of antiviral drugs strongly shape prescription behavior. At present, many low and middle income countries (e.g. Indonesia, Philippines, Vietnam) have low reimbursement coverage. The cheapest drug lamivudine is often prescribed. This results in a large population of patients with antiviral drug resistance. In China, most patients are on lamivudine or adefovir due to the low drug cost. A large-scaled surveillance of drug resistance has shown that 43% of patients on lamivudine and 8% of patients on adefovir have ge-notypic resistance to these drugs.31 Furthermore, a significant number of patients are put on sequential antiviral therapy and multi-drug resistant HBV mutants have emerged. Hong Kong, Taiwan and Korea adopt partial reimbursement policy. Patients may have free antiviral drugs if they fulfill certain criteria. Owing to the financial situation of the health authority, these criteria are usually more stringent than the regional treatment guidelines. In a report from Hong Kong in 2008, 40% of patients who were indicated for treatment did not receive anti-viral therapy for financial reason, and 83% of the treated patients were purchasing their own medication.32 In Taiwan, reimbursement is provided for a limited period of 1 year before 2009 and 3 years at present by the National Health Insurance Policy. After that, the patients have to pay for further treatment or stop treatment prematurely. This has led to high rate of disease relapse and clinical deterioration due to premature cessation of antiviral treatment.33
Therefore, cost-effectiveness analysis on the use of antiviral therapy is very important in the implementation of reimbursement policy. Entecavir and tenofovir are generally recommended as the first-line treatment in America and Europe due to their high antiviral potency and low risk of drug resistan-ce,34,35 and the benefit of tenofovir is also confirmed by cost-effectiveness analysis in Europe.36 However, the cost of drugs differs greatly in different geographical regions. For example, entecavir and tenofovir are much more expensive than lamivudine and telbi-vudine in most middle and low income countries. A cost-effectiveness analysis across the Asia-Pacific re-gion has demonstrated that the use of telbivudine as the first line agent and tenofovir for suboptimal on-treatment responders and drug resistance might be the most cost-effective approach in terms of 2-year HBV DNA suppression.37
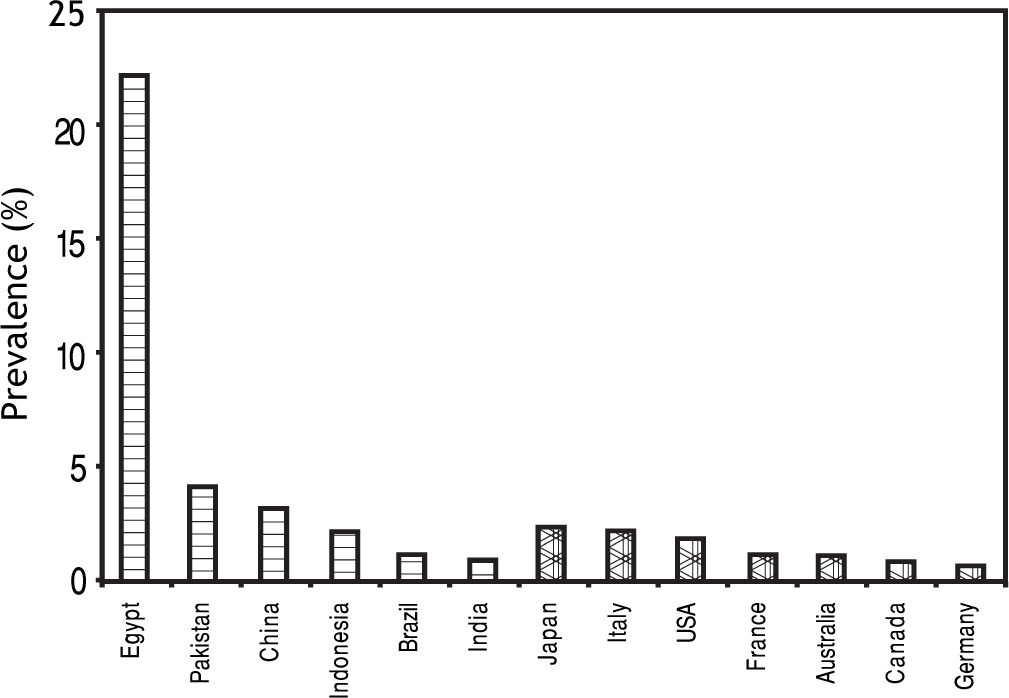
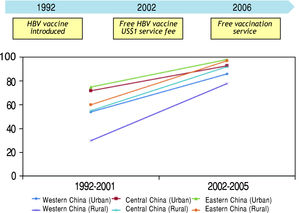
Hepatitis C Virus and HCCChronic infection with HCV affects more than 170 million people worldwide.38 In Western world, HCV is the leading cause of cirrhosis and HCC. Although the picture is overwhelmed by HBV in most developing countries, HCV infection is not uncommon in some areas (Figure 3).39
Seroprevalence of HCV infection in developing and developed countries.39
At present, HCV vaccine is still under development. Among patients with chronic hepatitis C, the standard treatment is peginterferon-alpha and riba-virin. After a course of treatment, sustained virolo-gical response (SVR), i.e. undetectable HCV RNA 6 months post-treatment, is achieved in 50 to 60% of cases.40 In a meta-analysis of 20 studies including 9 randomized controlled trials and 4,700 patients, antiviral therapy with interferon and/or ribavirin significantly reduces the risk of HCC development (risk ratio 0.43; 95% confidence interval 0.33, 0.56).41 The reduction in HCC, liver decompensation and cirrho-tic complications is primarily observed in patients achieving SVR.42
HCV genotype is one of the most important factors affecting the treatment response. In patients with genotypes 2 and 3 HCV infection, SVR can be achieved in 80 to 90% of treated patients. On the other hand, genotype 1 HCV infection is difficult to treat, with only 40 to 50% of patients responding to treatment. In Asia, the predominant genotype is genotype 1.43 Genotype 2 HCV is also commonly found in Taiwan and Japan, while genotype 6 HCV is common in Hong Kong and Vietnam. Egypt is unique in having a very high population prevalence of HCV infection and that genotype 4 is the predominant genotype. Although current treatment guidelines recommend similar treatment for genotypes 1, 4, 5 and 6, small studies from Asia suggest that the treatment response for genotype 6 HCV is better than that for genotype 1, with SVR in 70 to 80%.44,45 The response rate for genotype 4 is 50 to 60%.46
For years, it has been known that Africans with chronic hepatitis C respond less well to treatment than Caucasians, while studies from Asia consistently report the best results.47,48 There are a number of hypotheses to explain the observation. For examples, Asians were believed to have better drug adherence, smaller body size and less insulin resistance. Recent genomic studies, however, revealed that genetic factor is actually the main reason underlying the difference. Three independent genome wide association studies showed that gene polymorphism near the IL2B gene, which encodes interferon-A-3, is a strong predictor of treatment response in patients with chronic hepatitis C.49–51 Interestingly, the favorable allele associated with high rate of SVR is found in over 90% of the Chinese population but less than half in Africans.52,53
One main challenge to the current standard of care using peginterferon and ribavirin includes su-boptimal response rate among genotype 1 HCV infected patients. The situation is expected to be revolutionized by the introduction of direct acting antivirals. Phase 3 studies confirmed that the addition of HCV protease inhibitors boceprevir or tela-previr significantly increases the rate of SVR and reduces virological relapse in both treatment-naïve and previously treated patients with genotype 1 HCV infection.54–57 Furthermore, interferon-free regimens using combination of different directly acting antivirals are under active development.58 This will open new treatment opportunities for patients not tolerating or with contraindications to interfe-ron.59 However, these direct acting antiviral agents will be very expensive and impose heavy financial burden to low and middle economic countries. More studies are required before recommendation can be made on the use of direct antiviral agents in these regions, particularly among patients who has favorable IL28B genotype and early virological response to the standard peginterferon and ribavirin combination therapy. In a recent cost-effectiveness analysis in America, telaprevir inclusive regime is not cost-effective as initial therapy as compared to standard of care among patients with genotype 1 HCV infection and the CC IL28B genotype.60
Three large randomized controlled trials (HALT-C, COPILOT and EPIC3) addressed the question of whether low dose maintenance peginterferon treatment may reduce the risk of HCC in chronic hepatitis C patients with advanced liver fibrosis or cirrhosis who did not achieve SVR with standard therapy.61–63 None of the trials showed any difference in the incidence of HCC in treated and untreated patients up to 4 years of follow-up. On long-term follow-up up to a median of 6.1 years in the HALT-C trial, patients with baseline liver cirrhosis treated with peginterfe-ron had a delayed reduced incidence of HCC by approximately 50% as compared to the untreated controls.64 In view of the side effects and costs of extended peginterferon treatment, more data on the efficacy and cost-effectiveness on will be needed before long-term maintenance peginterferon treatment can be recommended for non-responders and relapsers.
AflatoxinAflatoxin is another risk factor of HCC in humid countries favoring the growth of Aspergillus flavus and Aspergillus parasiticus. In those areas, elevated urinary aflatoxin B1 metabolites doubles the risk of HCC.65 Anatoxin induces the transversion G→T in co-don 249 of the p53 tumor suppressor gene in human hepatocytes.66 In human HCC tissues, the presence of aflatoxin DNA adducts correlate with p53 mutations and p16 methylation.67 It is possible to reduce afla-toxin in food products by dry storage conditions.68
HCC SurveillanceThe measures described in previous sections aim at preventing new HBV and HCV infection (primary prevention) and preventing HCC development in at risk individuals (secondary prevention). The aim of HCC surveillance programs is to identify early cancers so that the patients may still undergo curative treatment and achieve complete remission.
The largest randomized controlled trial testing the efficacy of HCC screening was conducted in urban Shanghai, China.69 The study cluster randomized patients with chronic hepatitis to HCC screening (n = 9,373) or no screening (n = 9,443). Patients in the screening group had alpha fetoprotein testing and ultrasound examination every 6 months. More resectable HCC were identified in the screening group. Besides, among patients with HCC, 5-year survival rate was 46% in the screening group and 0% in the control group.
In real life clinical practice, the majority of HCC patients cannot undergo curative treatment because of advanced tumor staging and poor liver function.70 However, HCC identified by regular surveillance are typically smaller and fewer in number.71–73 As a result, more patients can undergo surgery or local ablative therapy, and achieve better survival.
Ultrasound examination and alpha fetoprotein testing are labor-intensive and costly.74 Most developing countries cannot afford indiscriminate surveillance of all patients with chronic liver diseases. Studies have showed that HCC surveillance would be more cost-effective if the screening tests are sensitive in detecting small HCCs, the incidence of HCC is high, and curative treatments are availa-ble.75 Therefore, the surveillance program should focus on patients at high risk of HCC development and be supported by effective referral and provision of treatment. For example, all patients who have liver cirrhosis, which is the most important risk factor for HCC, should undergo regular HCC surveillance unless the candidate has decompensated disease and is not suitable for liver transplanta-tion.76 As liver biopsy may not be feasible or acceptable to all patients for the diagnosis of early liver cirrhosis, non-invasive measure of liver fibrosis such as transient elastography may be useful in this setting.77–79
In chronic hepatitis B, a number of clinical, biochemical and virological factors have been shown to be associated with HCC risk in addition to liver cirrhosis. Long-term epidemiological studies have established the role of positive HBeAg, high HBV DNA and viral genotypes in HCC development.24,80 In a study of 3,653 chronic hepatitis B patients in Taiwan (REVEAL study), the incidence of HCC was 108 per 100,000 person-years for an HBV DNA level of < 300 copies/mL and 1,152 per 100,000 person-years for an HBV DNA level of 1 million copies/mL or above at a mean follow-up of 11.4 years.23 Genotype C HBV, particularly subgenotype Ce (prevalent in East Asia such as Korea, Japan and Northern part of China), is associated with a higher risk of HCC than genotype B HBV.24,81,82 Incorporating different risk factors, investigators have derived and validated various combined prediction scores for HCC development83–86 (Table 1). There is increasing evidence that the level of HBsAg can reflect the host immune control of the HBV.87 In an a post-hoc analysis of the REVEAL study, the level of serum HBsAg can further stratify the risk of HCC among patients who have HBV DNA < 10,000 copies/mL.88
Scoring systems to predict HCC risk in chronic hepatitis B patients.
| Author/Year | Population | Follow-up | Score | Performance |
|---|---|---|---|---|
| Yuen, et al. 2009.83 | 820 chronic hepatitis B patients at a hospital clinic. | 77 months | 16 * sex (male =1, female = 0) + age + 3 * HBV DNA (log copies/mL) + 19 * core promoter mutations (mutant = 1, wild type = 0) + 30 * cirrhosis (presence = 1; absence = 0). | AUROC: 0.88 at 5 years, 0.89 at 10 years. At a cutoff of 101 points, the sensitivity and specificity for HCC was 84 and 76% at 5 years, and 88 and 79% at 10 years, respectively. |
| Wong, et al. 2010.84 | 1,005 chronic hepatitis B patients in an HCC surveillance project (training cohort); 424 patients in a hospital clinic (validation cohort). | 10 years | Age (> 50 years = 3 points; ≤ 50 years = 0) + albumin (≤ 35 g/L = 20 points; > 35 g/L = 0) + bilirubin (> 18 μmol/L = 1.5 points; ≤ 18 umol/L = 0) + HBV DNA (> 6 log copies/mL = 4 points; 4-6 log = 1 point; ≤ 4 log = 0) + cirrhosis (yes = 15 points; no = 0) | AUROC 0.76 at 5 years and 0.78 at 10 years. At a cutoff of 5 points, the sensitivity and specificity were 78 and 73% at 5 years, and 81 and 76% at 10 years, respectively. |
| Yang, et al. 2010.85 | Chronic hepatitis B patients from 7 Taiwan townships randomly assigned to the derivation cohort (n = 2,435) and validation cohort (n = 1,218). | 11.4 years | Sex (male = 2 points; female = 0) + age (1 point for every 5 years) + family history of HCC (yes = 2 points; no = 0) + alcohol consumption (yes = 1 point; no = 0) +ALT (< 15 U/L = 0; 15-44 = 1 point; ≥ 45 = 3 points) + HBeAg (positive = 3 points; negative = 0). (Alternative models incorporating HBV DNA and genotypes available). | AUROC 83% at 5 years and 82% at 10 years. |
| Yang, et al. 2011.86 | 3,584 non-cirrhotic patients from Taiwan (development cohort) and 1,505 patients in 2 Hong Kong and 1 South Korea centers (validation cohort). | 12.0 years (development cohort); 6.3, 9.4 and 7.0 years (3 validation cohorts). | A 17-point score comprising of 5 variables: sex, age, ALT, HBeAg and HBV DNA. | AUROC 78% at 5 years and 81% at 10 years. |
ALT: alanine aminotransferase. AUROC: area under the receiver operating characteristics curve. HBV: hepatitis B virus.
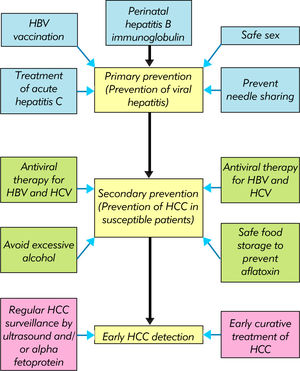
The development of HBV vaccine and antiviral therapy against HBV and HBV has revolutionized the management of chronic viral hepatitis and resulted in dramatic improvements in clinical outcomes. Together with general preventive measures, the number of patients affected by viral hepatitis and cirrhotic complications is expected to decline. HCC surveillance further allows detection of early cancers and provision of curative therapy. In the near future, the key is to ensure availability and affordability of these measures to people in low and middle income countries (Figure 4).
Competing InterestsVincent Wong has served as an advisory committee member for Roche, Novartis, Gilead and Otsuka. He has also served as a speaker for Bristol-Meyers-Squibb, Roche, Novartis, Abbott Diagnostics and Echosens. Henry Chan is a consultant for Abbott, Bristol Myer Squibb, Gilead, Merck, Novartis and Roche, and has received honorarium for lecture for Abbott, Bristol Myer Squibb, Echosens, Gilead, Glaxo-Smith-Kline, Merck, Novartis and Roche.
Abbreviations- •
HBeAg: hepatitis B e antigen.
- •
HbsAg: hepatitis B surface antigen.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
SVR: sustained virological response.