Introduction. Hepatitis C virus genotype 4 is predominant in the Middle East and Northern Africa, even if it has recently spread to Southern Europe. Data about the treatment of post-liver transplantation (LT) genotype 4 hepatitis C recurrence are scarce. We report a retrospective analysis of post-LT genotype 4 hepatitis C treatment in 9 Italian transplant centres, focusing on the overall survival rates and treatment outcome.
Results. Among 452 recipients, we identified 17 HCV genotype 4 patients (16 males, 1 female) transplanted between 1998 and 2007. All patients received combined antiviral treatment with conventional doses of interferon (recombinant or pegylated) and ribavirin after histological diagnosis of hepatitis C recurrence. The observed overall survival after LT was 100% at 1 year and 83.3% at 5 years. More than 1/3 (35.3%) of patients achieved a sustained virological response (SVR) and 40% (data available in 15 subjects) an early virological response (EVR), which was significantly associated with the achievement of SVR (overall accuracy: 85.7%; predictive values of EVR absence/presence 80/88.8%; chi-square p < 0.05).
Conclusion. In conclusion, in post-LT genotype 4 hepatitis C treatment, SVR rates are similar to genotype 1. Patients who don’t show an EVR are not likely to achieve a SVR.
Hepatitis C virus (HCV) has 6 major genotypes and over 76 subtypes.1,2 Genotype 4 is widely prevalent in the Middle East and Northern Africa and is predominant in Egypt, where accounts for more than 90% of HCV infections.3,4 Genotype 4 HCV infection has a low prevalence in Europe, ranging between 0 and 19%, even if, in Mediterranean countries such as France, Spain, Greece and Italy, a trend towards an increasing prevalence has been recently observed.5,6 The main reasons for this phenomenon appear to be the increased immigration from high prevalence areas and the diffusion among intravenous drug abusers.
Early reports have shown that patients with genotype 4 chronic hepatitis C respond as poorly as those with genotype 1 to conventional interferon (IFN) alpha and to combined IFN alpha plus ribavirin therapy.7–10 More recently, a few trials on the treatment of genotype 4 chronic hepatitis C using pegylated (PEG) IFN alpha 2b or alpha 2a plus ribavirin have been published, reporting highly variable rates of sustained virological response (SVR), ranging from 33 to 69%.11–17 On the whole, the SVR rate of patients with chronic genotype 4 hepatitis C treated with PEG-IFN plus ribavirin seems to be slightly higher than that observed for genotype 1 (42-46%), but lower than that observed for genotypes 2 and 3 (76-82%).18,19 Furthermore, recent data suggest that, in genotype 4 patients, the achievement of a rapid virological response (RVR).14,20 or an early virological response (EVR)21 may be highly predictive of a subsequent SVR; moreover, a higher SVR rate can be observed in Egyptian patients compared to matched European patients.15
Only a few data about the clinical course and the treatment outcome of patients with genotype 4 chronic hepatitis C recurrence after liver transplantation (LT) are still currently available, especially in Europe.
The aim of this study is to retrospectively evaluate the effectiveness of the antiviral treatment in an Italian multicentric cohort of patients with genotype 4 chronic hepatitis C recurrence after LT.
Material and MethodsSeventeen patients (16 males and 1 female) aged from 39 to 59 years and affected by genotype 4-related chronic hepatitis C recurrence after LT were enrolled in the study. Five of them were native to Egypt, the remainders were Caucasian. All patients were followed-up in 9 Italian Transplant centres, during the period 1996-2010; and the time between LT and the clinical recurrence of hepatitis C ranged from 4 to 67 weeks (mean 25.1 weeks). None of the investigated patients had experienced a pre-LT treatment for HCV infection.
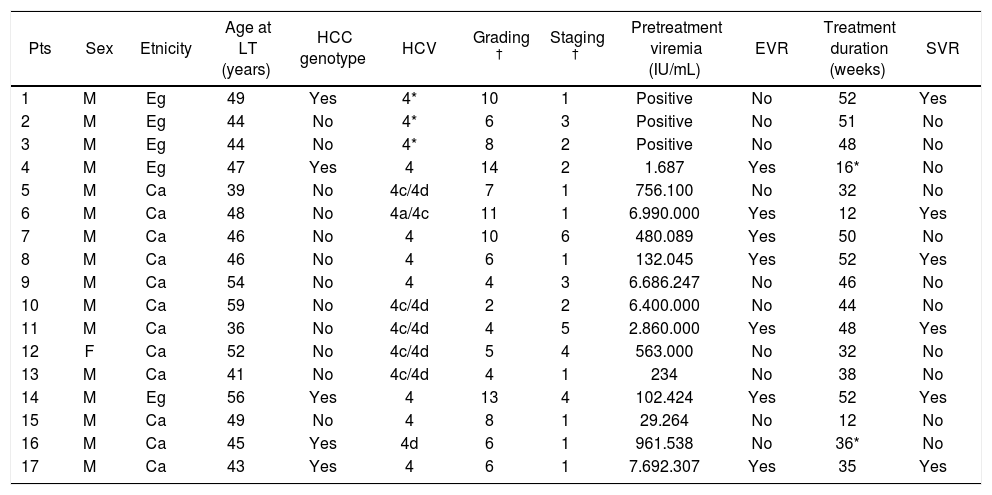
The outstanding clinical characteristics of the investigated patients are summarized in table 1.
Demographic and clinical features of the 17 investigated patients.
| Pts | Sex | Etnicity | Age at LT (years) | HCC genotype | HCV | Grading † | Staging † | Pretreatment viremia (IU/mL) | EVR | Treatment duration (weeks) | SVR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Eg | 49 | Yes | 4* | 10 | 1 | Positive | No | 52 | Yes |
| 2 | M | Eg | 44 | No | 4* | 6 | 3 | Positive | No | 51 | No |
| 3 | M | Eg | 44 | No | 4* | 8 | 2 | Positive | No | 48 | No |
| 4 | M | Eg | 47 | Yes | 4 | 14 | 2 | 1.687 | Yes | 16* | No |
| 5 | M | Ca | 39 | No | 4c/4d | 7 | 1 | 756.100 | No | 32 | No |
| 6 | M | Ca | 48 | No | 4a/4c | 11 | 1 | 6.990.000 | Yes | 12 | Yes |
| 7 | M | Ca | 46 | No | 4 | 10 | 6 | 480.089 | Yes | 50 | No |
| 8 | M | Ca | 46 | No | 4 | 6 | 1 | 132.045 | Yes | 52 | Yes |
| 9 | M | Ca | 54 | No | 4 | 4 | 3 | 6.686.247 | No | 46 | No |
| 10 | M | Ca | 59 | No | 4c/4d | 2 | 2 | 6.400.000 | No | 44 | No |
| 11 | M | Ca | 36 | No | 4c/4d | 4 | 5 | 2.860.000 | Yes | 48 | Yes |
| 12 | F | Ca | 52 | No | 4c/4d | 5 | 4 | 563.000 | No | 32 | No |
| 13 | M | Ca | 41 | No | 4c/4d | 4 | 1 | 234 | No | 38 | No |
| 14 | M | Eg | 56 | Yes | 4 | 13 | 4 | 102.424 | Yes | 52 | Yes |
| 15 | M | Ca | 49 | No | 4 | 8 | 1 | 29.264 | No | 12 | No |
| 16 | M | Ca | 45 | Yes | 4d | 6 | 1 | 961.538 | No | 36* | No |
| 17 | M | Ca | 43 | Yes | 4 | 6 | 1 | 7.692.307 | Yes | 35 | Yes |
* Non-Pegylated IFN. According to Ishak score. M: male. F: female. Eg: Egyptian. Ca: Caucasian. HCC: hepatocellular carcinoma. EVR: early virologic response. SVR: sustained virologic response.
The quantitative value of pre-treatment HCVRNA was available in 14 out of 17 patients (mean viremia 2.423.235 IU/mL, range 234-7.692.307 IU/mL), and a high viral load (more than 600.000 IU/mL) was detected in 7/14 cases. In the remaining 3 patients, transplanted before 2000, only a positivity for serum HCV-RNA was available.
Hepatocellular carcinoma (HCC) before LT was diagnosed in five patients (29.4%).
Patients were treated with conventional doses of recombinant (2 cases) or PEG-IFN (15 cases) alpha plus ribavirin for a scheduled period of 48-weeks.
ResultsThe main results of the study are shown in table 1. We did not find any significant difference in the immunosoppressive regimen scheduled for these patients, since 8 received cyclosporine, 8 tacrolimus and 1 rapamcycine as the main immunosuppressive agent. The mean donor age was 46.6 (16-86 yrs); donors were 9 males and 8 of females; a donor-recipient gender mismatch was present in 7 case, always of the type female donor in male recipient.
Antiviral treatment duration was of at least 24 weeks in 14 subjects (82.3%), and 8 of them (47%) completed a standard 48-week course of treatment. Three patients received less than 24 weeks of antiviral treatment; in particular, patient n. 4 withdrew the treatment at week 16 for urgent abdominal surgery due to laparocele, while patients n. 6 and n. 15 suspended the treatment after 12 weeks for personal choice and for severe anemia, respectively. Six patients withdrew the treatment after 24 weeks, for the occurrence of neuropsychiatric symptoms, severe neutropenia, cholangitis, hepatocellular carcinoma recurrence and, finally, in two cases, lack of virological response.
A SVR was achieved in more than one third (35.3%) of the investigated patients, with a negative HCV-RNA persisting for at least 3 years (long term response) in all of them.
An EVR was achieved in 7 out of the 17 patients (41.1%). In the 14 patients who completed at least 24 weeks of treatment, we could also assess the ability of EVR in predicting the occurrence of SVR; 80% (4/5) of the patients exhibiting an EVR achieved a SVR, while it occurred in only one patient without EVR. In this way, EVR was significantly associated with the occurrence of SVR (overall accuracy: 85.7%; predictive values of EVR absence/presence 80/88.8%; chi-square p < 0.05).
Interestingly, one of the 3 patients who did not complete a 24-week course of treatment (Table 1, patient n. 6), achieved an EVR together with a SVR.
The observed overall survival rates (Kaplan-Meier method) were 100% at 1 year and 83.3% at 5 years after LT. No significant differences were found among the survival rates of patients with or without SVR, probably due to the low number of investigated subjects.
DiscussionEnd stage HCV-related liver cirrhosis is the most common indication for LT in Europe.22 However, HCV reinfection of the graft occurs almost universally, leading to graft cirrhosis within 5 years after LT in 10-30% of recipients and to an impaired survival.23 At present, the gold-standard of antiviral therapy is the combination of PEG-IFN plus ribavirin, administered, in most centres, after the histological diagnosis of infection recurrence in the graft. However, the reported SVR rates that could be achieved with the combined treatment are significantly lower than 50%, least of all in genotype 1 patients.24–26
Similarly to immunocompetent subjects with chronic hepatitis C, SVR rates in transplanted patients are significantly lower in genotypes 1 and 4 (25-40%) than in genotypes 2 and 3 (53-76%).24–29 Despite the increasing diffusion of genotype 4 HCV in the Mediterranean countries, only a few data about its response to antiviral treatment after LT are available so far.4 Selzner, et al.24 treated with conventional or PEG-IFN 119 patients with genotype 1 and 4 recurrent hepatitis C after LT; SVR was achieved in 40% of cases but no data about the response of the 9 patients with genotype 4 HCV infection were reported. Similarly, Jimenez-Perez, et al.30 reported a 41% rate of SVR in 41 patients transplanted for recurrent hepatitis C; only in 4 cases graft reinfection was due to HCV genotype 4, but no data about their response to treatment were provided. The only available literature series specifically concerning transplanted patients with recurrent genotype 4 chronic hepatitis C is those published by Mudawi, et al.,31 who treated 17 patients from Saudi Arabia with a 48-week course of PEG-IFN alpha 2a plus ribavirin, achieving a SVR in 7 of them (41%) and Al-Hamoudi, et al.,32 who treated 25 patients from Saudi Arabia with a 48-week course of PEGIFN alpha 2a plus ribavirin, achieving a SVR in 14 of them (56%).
Therefore, our study, despite the small number of enrolled patients, represents to our knowledge the largest available series in Europe regarding post-LT genotype 4 recurrent hepatitis C antiviral treatment.
According to the rates reported in patients with genotype 1, a SVR was achieved in 35.3% of our patients with recurrent genotype 4 chronic hepatitis C. Overall, EVR was able to predict the occurrence of SVR (p < 0.05) in 80% of patients with more than 24 weeks of therapy, while, in absence of EVR, a SVR was achieved only in one case. The SVR rate reported by our study is lower than that reported by Al-Hamoudi, et al.,33 mainly because the authors excluded difficult-to-treat patients (those who failed previous pre-or post-transplant treatment, with renal failure, and pretreatment cytopenia), reporting only one case of treatment withdrawal. Moreover, in immunocompetent patients, Egyptian origin has been reported to be associated to higher SVR probabilties. Although the number of our transplanted patients is too low to draw any firm conclusion, we were not able to find a statistical difference (Chi-square 0.07; 1 d.f.) between SVR rates among Egyptian patients (40%) and the Italian ones (33.3%) (Table 1).
It is well known that, in immunocompetent subjects with genotype 1 chronic hepatitis C, the absence of EVR is a very strong predictor of treatment failure, since more than 90% of patients who do not reach EVR fail to achieve SVR.33 Some recent papers confirmed the relevance of EVR in predicting a subsequent SVR also in transplanted patients undergoing antiviral treatment for recurrent hepatitis C, mostly genotype 1-related.24,25,28 Al-Hamoudi, et al.3 also confirmed that the probability of achieving SVR was related to achieving a negative HCV RNA at week 12. Our experience supports this evidence also in genotype 4 transplanted patients; the-refore, in the subgroup of genotype 4 patients treated after liver transplantation who do not achieve an EVR, treatment discontinuation may be considered.
Literature data highlight that, independently from HCV genotype, the achievement of SVR seems to improve significantly the long term survival of patients with post-LT recurrent hepatitis C undergoing antiviral treatment.27,34 However, our series cannot support these data, since we were not able to find a statistical difference between the cumulative survival of subjects with or without SVR, probably due to the relatively low number of investigated patients. Furthermore, we included in our retrospective multicentric series only genotype 4 patients who were treated for HCV gen 4 recurrence after liver transplantation. Unfortunately, due to the study design, we were not able to collect data about those patients who did not receive the treatment, either due to mild hepatitis C recurrence or to contraindications to combined antiviral treatment. Therefore, we cannot provide any comparison regarding survival of these two groups.
As a last consideration, a recent meta-analysis reported that more than 84% of Egyptians with HCC are positive for genotype 4 HCV.35 In our series, HCC was diagnosed in five patients (29.4%) and three of them were Egyptians. Then, HCC was present in 3 out of 5 Egyptian patients (60%) and in only 2 out of 12 Caucasians (16.7%). In particular, one of the 5 patients with HCC achieved a SVR but died 8 months after the end of the antiviral treatment due to HCC recurrence. However, in the other 4 cases, no HCC recurrence was observed. However, the small number of patients included in our retrospective series does not allow us to draw any epidemiologic conclusion.
ConclusionIn conclusion, our study shows that transplanted patients with genotype 4 recurrent hepatitis C undergoing antiviral treatment achieve SVR in about one third of cases, and that EVR is a strong predictor of subsequent SVR.